It is usually administered as a single agent and has a clinical response of 5 to 10 percent. Part of the major problem is that the Melanoma Cells can become chemical resistant to the therapy because the cells can morph based on their microenvironment.1
The United States Food and Drug Administration (FDA) officially recognized the efficacy of Interluekin -2 (IL-2) in patients with metastatic melanoma by granting approval for commercial marketing of the drug for that indication in 1998, based on tumor response and survival data generated from clinical trials of the intermittent high-dose bolus regimen.
Today, we are in a new era of therapy. Immunotherapy has come to the forefront of Melanoma Therapy. Researchers are beginning to crack the code and are making great strides inmmunotherapy.
To be able to unravel the “Melanoma Maze” as I call it, we need to know “How Melanoma works.” In this next section, It may get very detailed, but I will try to explain with the help of the experts and a dictionary. I am learning like you so please bear with me.
So here it goes!!!!!
Cancer begins in cells, the building blocks that make up tissues. Tissues make up the organs of the body. Normally, cells grow and divide to form new cells as the body needs them. When cells grow old, they die, and new cells take their place.
Sometimes this orderly process goes wrong and new cells form when the body does not need them, and old cells do not die when they should. These extra cells can form a mass of tissue called a growth or tumor. Not all tumors are cancer.
In Melanoma, the cause is by the uncontrolled, unregulated growth of Melanocytes. The cell cycle proceeds unregulated, and cell growth proliferates.
It has been suggested that there are Five Stages to Metastatic Melanoma .2
Five stages of tumor progression have been suggested:
1) Benign melanocytic nevi- Melanocytic nevi are benign neoplasms or hamartomas composed of melanocytes, the pigment-producing cells that constitutively colonize the epidermis. The malignant analogue of a melanocytic nevus is melanoma. This where Melanoma can start, but can also start at eyes, ears, GI tract, leptomeninges, and oral and genital mucous membranes and even sometimes the primary site is unknown and is called “Occult Primary Melanoma”.
2) Melanocytic nevi with architectural and cytologic atypia (dysplastic nevi) “An atypical nevus, usually larger than 5 millimeters in diameter with variable pigmentation and ill-defined borders, marked by melanocytic dysplasia and associated with an increased risk for the development of nonfamilial cutaneous malignant melanoma. Also called dysplastic nevus3” The Precursor to melanoma.
“The sequence of events in which normal melanocytes transform into melanoma cells, referred to as melanomagenesis, is poorly understood. It likely involves a multistep process of progressive genetic mutations that (1) alter cell proliferation, differentiation, and death and (2) impact susceptibility to the carcinogenic effects of ultraviolet radiation.4”
3) Primary malignant melanoma, radial growth phase
This is early pattern of growth of cutaneous malignant melanoma in which tumor cells spread laterally into the epidermis. This is where if it is identified and removed with surgery, there is 95 % cure rate. The Melanoma Cells have not entered into the lymphatic system.
4) Primary malignant melanoma, vertical growth phase
The late pattern of growth of cutaneous malignant melanoma in which tumor cells spread from the epidermis into the dermis.

Figure 1. Human Skin5
5) Metastatic malignant melanoma
Each step in tumorigenesis is marked by a new clone of cells with growth advantages over the surrounding tissues. The cancer, as it invades in its place of origin, may also work its way into blood vessels. If this occurs, it provides yet another route for the cancer to spread to other organs of the body. When the cancer spreads elsewhere in the body, it has become systemic in extent and the tumor growing elsewhere is known as a metastasis.
What are the different Types of Malignant Melanoma?
1) Superficial spreading melanoma
2) Nodular melanoma
3) Lentigo maligna melanoma
4) Acral melanoma.
5) Ocular Melanoma
Superficial spreading melanoma does just as it sounds. It is on the surface of your skin and spreads horizontally. It is the most common and is a bout 65% of the Melanoma reported cases.
Nodular melanoma is a much less common form of melanoma. It usually starts as a raised lesion that is dark black-blue or bluish-red, however some can lack color. Nodular melanomas account for approximately 15% of cases. It is fast spreading and usually grows vertically in both directions.
Lentigo maligna melanoma is usually found on the palms of the hands, soles of the feet and/or around the toenails. 5-15% of all cases
Acral melanoma is an uncommon type of melanoma. It is the most common type seen in nonwhite individuals. It usually occurs on the palms and soles. Sometimes it occurs on the vulva and vagina. Acral melanoma accounts for 5% of Melanoma cases.
Ocular melanoma occurs in the eye and is estimated that 2% of the total melanoma diagnosed per year is ocular.
So where do we go from here?
Since we now know that the cause for this unregulated growth of Melanocytes is mutation in certain genes, we need to know the signaling pathways that cells took to grow. So researchers like Dr. Smalley, Dr Herlyn and many others have recently identified that the activating mutation in the BRAF gene on chromosome 7 linked to 60% of the cases in Melanoma.6
“This gene encodes a protein belonging to the raf/mil family of serine/threonine protein kinases. This protein plays a role in regulating the MAP kinase/ERKs signaling pathway, which affects cell division, differentiation, and secretion.
Mutations in this gene are associated with cardiofaciocutaneous syndrome, a disease characterized by heart defects, mental retardation and a distinctive facial appearance. Mutations in this gene have also been associated with various cancers, including non-Hodgkin lymphoma, colorectal cancer, malignant melanoma, thyroid carcinoma, non-small cell lung carcinoma, and adenocarcinoma of lung.”7
Our understanding of B-RAF regulation was greatly increased recently when the crystal structure of the B-RAF kinase domain bound to the small molecule inhibitor BAY43-9006 was solved (Wan et al., 2004).
So in 2006, in Cancer Research 66, 1611-1619, February 1, 2006], A research paper came out entitled: The Raf Inhibitor BAY 43-9006 (Sorafenib) Induces Caspase-Independent Apoptosis in Melanoma Cells. David J. Panka1, Wei Wang2, Michael B. Atkins1 and James W. Mier1
1 Division of Oncology, Beth Israel Deaconess Medical Center and Harvard Medical School and 2 Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston, Massachusetts
The paper caused excitement among researchers in that Raf Inhibitor was a way to block the pathway and cause apoptosis in Melanoma. This is a form of cell death in which a programmed sequence of events leads to the elimination of cells without releasing harmful substances into the surrounding area.
So what are the signaling pathways known to date?
Figure 2. Signaling pathways affected by the common primary changes in human melanoma, and some inter-relationships8
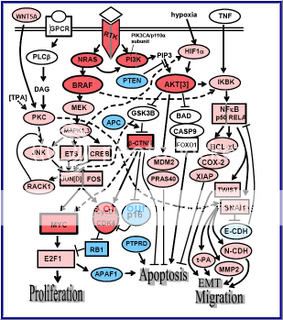
“Molecules shown in bright red are those clonally amplified or activated in some melanomas; those in bright blue are clonally deleted or inactivated in some melanomas. Pale red and pale blue indicate proteins that are at least secondarily upregulated or downregulated in melanoma, respectively. Rectangles indicate transcription factors. Some components have a larger outline; this is just to emphasize apparently important signaling nodes, and does not reflect molecular size.”
Attempts have been made to focus on pathways known to be present and active in melanoma cells (Dr. Smalley, Dr. Herlyn), although some sections of pathways are derived from the broader literature, and with the help of the pathway maps of Weinberg.9
Figure 3. Schemic of known active signaling pathways in Melanoma
by Dr. Smalley and Dr. Herlyn6
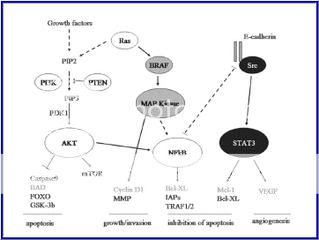
As you can see, from the diagrams, some of these pathways are intertwined and make the research more difficult but not impossible. Careful planning of the experimentations may yield new target therapies down the road. There may be a combo of Chemo and Immunology together to stabilize the Melanoma Beast.
So Now, Where does the Patients come into play?
We need to start by having our Blood typed for Biomarker. A biological marker, or biomarker, is a phenotypic parameter (eg, a substance, structure, or process) that is measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. This step One. Step two is the biopsy of your tumor and getting it typed/mapped. If the Oncologist can get the pathway that is mutated, They will be able to direct to the right therapy base on that mutation.
Example: Kit Mutation
“THURSDAY, April 17 2008 (HealthDay News) -- The cancer drug Gleevec has forced metastatic melanoma into remission for the first time, report researchers at the Dana-Farber Cancer Institute in Boston.
The case involves a 79-year-old woman with melanoma tumors in several parts of her abdomen. The tumor cells carried an abnormality in a gene called KIT, so the patient was enrolled in a clinical trail of the drug imatinib (Gleevec), which targets the KIT gene.
Four weeks after the woman started therapy, there was dramatic reduction in tumor size and metabolism. Two of the tumor masses had vanished, and several others were much smaller. After four months, the tumors were still in check and, nine months later, the women was still taking the drug and her condition remained stable.
The report was published in the April 20 issue of the Journal of Clinical Oncology .
"This is the first proof of principle that we can find an Achilles' heel in melanoma and by targeting that gene with a drug, cause the [tumor cells] to die. It is especially exciting because there haven't been any effective treatments for melanoma patients with metastatic disease," study author Dr. Stephen Hodi said in a prepared statement.
He said this case may involve just one patient, but should inspire new hope in the fight against melanoma. Because previous research failed to identify any genetic weak point that could be targeted to stop melanoma cell growth, some researchers believed that no such Achilles' heel existed for melanoma cells. The discovery of this one suggests there may be others.”
SOURCE: Dana-Farber Cancer Institute, news release, April 17, 2008
I believe we are now able to use the molecular profile of disease to deliver the right therapy to the right patient at the right time. But we must first build the infrastructure that will support Targeted Therapy with Personalized Medicine. This will require a Novel approach to the Global Healthcare seen. We would need a seamless communication along with various components including a national tissue repository, molecular diagnostic standards and procedure in place. A network of clinical sites working in collaboration to identify cohorts of patients with similar abnormal molecular profiles that is in a database and is available to Oncologists and Medical Researchers alike, a bioinformatic infrastructure integrating all of these elements.
It may be already here!!!!!!
Big Changes at BiobankCentral
Posted: 27 Jan 2009 10:36 AM CST
by Kate Blenner, Program Analyst, FasterCures10
We at FasterCures are very pleased to announce some big changes to BiobankCentral.org, the Web site we have established to highlight the importance of biobanks to medical research. This site links researchers to resources, encourages the donation of specimens, and educates the public about the benefits of research on banked biospecimens. After interviewing key stakeholders, including patient advocates, biobank operators, and leaders in the field of biospecimen research, we will begin staging some new features and functions that will make Biobankcentral.org even more useful to visitors hoping to learn more about these critical resources.
The first of these new features is the Spotlight on Innovation which will highlight individuals and organizations doing exceptionally innovative work in biobanking. Our first Spotlight focuses on the Susan G. Komen for the Cure® Tissue Bank at the Indiana University Simon Cancer Center, or Komen Tissue Bank (KTB) for short. This bank’s mission is to collect samples of normal, healthy breast tissue and other biospecimens from healthy women for breast cancer research. Yes, you read that correctly—normal tissue. Healthy women.
In its 1998 priorities for cancer research, the National Cancer Institute identified the lack of knowledge about the normal biology and development of the mammary gland as a significant barrier to finding a breast cancer cure. Most research to date has focused on characterizing diseased tissue, but without the frame of reference of how healthy tissue develops and functions opportunities for a cure could be missed. Complicating the issue was a shortage of normal tissue available for study. The NCI’s recommendations to address the ‘tissue issue’ languished for a few years, until some motivated advocates and clinicians at IU Simon Cancer Center decided to form the KTB.
Despite initial skepticism that healthy women would want to go through an invasive collection procedure, KTB put its faith in the motivation of the breast cancer advocacy community—and it paid off. They have collected thousands of samples to date, and communities across the country have asked KTB to set up its collection tent at their local Race for the Cure events. As bank co-founder and patient advocate Connie Rufenbarger told me: “These women have walked, they’ve written checks, they’ve lit candles—they’ve done everything they can to demonstrate they want to help. [The response] really speaks to the fact that there isn’t a whole lot you could ask that women wouldn’t give you to cure this disease.”
I hope you enjoy this first Spotlight of the Komen Tissue Bank as much as I enjoyed speaking with its remarkable founders and staff. If you have a moment, stop by the KTB Web site to find out how you can get involved in their work to find a cure. And, of course, keep an eye on BiobankCentral.org—we have many more exciting new changes to come.
References
1. Cedric Gaggioli and Erik Sahai- Melanoma invasion – current knowledge and future directions Pigment Cell Res. 2007 20; 161–172
2. http://www.clevelandclinicmeded.com/medicalpubs/
3. http://www.answers.com/topic/malignant-melanoma
4. Demierre MF, Nathanson L. Chemoprevention of melanoma: an unexplored strategy. J Clin Oncol. Jan 1 2003;21(1):158-65. [Medline]
5. http://www.mydr.com.au/files/images/categories/skinhair/skinstructure.gif
6. Keiran S.M. Smalley and Meenhard Herlyn Targeting Intracellular Signaling Pathways as a Novel Strategy in Melanoma Therapeutics Ann. N.Y. Acad. Sci. 1059: 1–10 (2005). 2005 New York Academy of Sciences.doi: 10.1196/annals.1339.005
7. http://www.genecards.org/cgi-bin/carddisp.pl?gene=BRAF
8. Dorothy C Bennett- How to make a melanoma: what do we know of the primary clonal events? Division of Basic Medical Sciences, St George's, University of London, Cranmer Terrace, London SW17 0RE, UK.
9. R. A. Weinberg The Biology of Cancer Garland Science, Taylor & Francis Group,. LLC, London, 2007, 864 pp., ISBN-10 0-8153-4078-8/
10. http://www.fastercures.org/





No comments:
Post a Comment