Vernon K. Sondak, MD
Chief, Division of Cutaneous Oncology
Director, Surgical Education
H. Lee Moffitt Cancer Center & Research Institute
Professor
Departments of Interdisciplinary Oncology and Surgery
University of South Florida College of Medicine
Tampa, FL
Fifth International Symposium on Melanoma
and Other Cutaneous Malignancies
New York, New York
March 7, 2008
LESSON 1
There has been no significant improvement in overall
survival for metastatic melanoma in the past 30 years
This Is a Hard Lesson to Swallow!!!!!!!!!!!!!!!!
Please don't shoot the messenger!!!!!!
The good news is the 1971 to 2001!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
So this is the new begining with targeted therapy I believe!!!!!!!
Lessons From Three Decades of Clinical Trials in Metastatic Melanoma
This is Jim Breitfeller's journey into the Maze of Melanoma. Jim Breitfeller has gathered medical information for the patient and the caregiver. As Lance Armstrong would say "Lets stand Up to Cancer" Jim's Battle with the Beast July 2005 to present.
Friday, January 30, 2009
Wednesday, January 28, 2009
State of the Science Lecture Series On Melanoma ..Jim Breitfeller
If you want, you can dive into these Lectures on Melanoma
http://www.webtie.org/SOTS/Meetings/Melanoma/05-05-2003/lectures.htm
State of the Science Lecture Series On Melanoma
Jimmy B
http://www.webtie.org/SOTS/Meetings/Melanoma/05-05-2003/lectures.htm
State of the Science Lecture Series On Melanoma
Jimmy B
Medicare Broadens Coverage of Cancer Drugs.. Melanoma..Jim Breitfeller
Medicare Broadens Coverage of Cancer Drugs
As Reported by The New York Times. 2009 Jan 26
As of November 2008, Medicare now covers cancer drugs prescribed for off-label uses not indicated by the Food and Drug Administration (FDA). This new policy continues to stir the controversy waged by proponents of the move, who say it helps patients receive the most up-to-date care and furthers research, and opponents, who cite cost and patients’ potential exposure to adverse effects from treatments that may not provide any benefit. There is no provision in the new policy for monitoring outcomes from off-label uses. How much the new policy will increase Medicare’s spending on cancer drugs above the $2.4 billion it spent in 2007 is difficult to know, because Medicare canceled a cost analysis of the changes. Gemzar (gemcitabine) and Avastin (bevacizumab) are 2 of the drugs that will receive expanded coverage. In 2007, Medicare rejected nearly all claims for off-label use of bevacizumab for ovarian cancer.
Medicare asserts that the new policy responds to oncologists’ criticisms that it has been too slow in the past in recognizing off-label uses of drugs that show clinical promise. The American Society of Clinical Oncology applauds the new rules. Oncologists have needed the flexibility to prescribe cancer drugs off label, singly or in combination, because FDA drug approvals can take years. The off-label use of thalidomide for multiple myeloma is a case example. Rules for coverage limiting patients to only a few drugs could deprive them of a life-saving treatment. Sometimes numerous drug trials are necessary before they find a treatment to which they respond.
Medicare relies on 3 compendia as reference guides in deciding which off-label uses of cancer drugs to cover. It will review its choice of compendia every year. The contents of the compendia wield considerable power in decision making under the new policy: as long as 1 of the 3 compendia recommends a particular cancer treatment, Medicare is obligated to pay for it.
Conflicts of interest related to ties between the compendia developers and the pharmaceutical industry are being examined in a report to be delivered to Medicare soon. Some experts contributing to the National Comprehensive Cancer Network (NCCN) guidelines, which Medicare uses, have ties to drug companies. Similarly, the American Hospital Formulary compendium, which Medicare consults, used to be published by a nonprofit group. It now operates through the Foundation for Evidence-Based Medicine, to which a drug company can pay a $50,000 fee to have its drug reviewed for inclusion in the compendium. Less than one-third of such reviews result in a recommendation, however.
Medicare officials acknowledge that various issues that still need to be resolved, but they stand behind their choice of compendia.
Jimmy B
As Reported by The New York Times. 2009 Jan 26
As of November 2008, Medicare now covers cancer drugs prescribed for off-label uses not indicated by the Food and Drug Administration (FDA). This new policy continues to stir the controversy waged by proponents of the move, who say it helps patients receive the most up-to-date care and furthers research, and opponents, who cite cost and patients’ potential exposure to adverse effects from treatments that may not provide any benefit. There is no provision in the new policy for monitoring outcomes from off-label uses. How much the new policy will increase Medicare’s spending on cancer drugs above the $2.4 billion it spent in 2007 is difficult to know, because Medicare canceled a cost analysis of the changes. Gemzar (gemcitabine) and Avastin (bevacizumab) are 2 of the drugs that will receive expanded coverage. In 2007, Medicare rejected nearly all claims for off-label use of bevacizumab for ovarian cancer.
Medicare asserts that the new policy responds to oncologists’ criticisms that it has been too slow in the past in recognizing off-label uses of drugs that show clinical promise. The American Society of Clinical Oncology applauds the new rules. Oncologists have needed the flexibility to prescribe cancer drugs off label, singly or in combination, because FDA drug approvals can take years. The off-label use of thalidomide for multiple myeloma is a case example. Rules for coverage limiting patients to only a few drugs could deprive them of a life-saving treatment. Sometimes numerous drug trials are necessary before they find a treatment to which they respond.
Medicare relies on 3 compendia as reference guides in deciding which off-label uses of cancer drugs to cover. It will review its choice of compendia every year. The contents of the compendia wield considerable power in decision making under the new policy: as long as 1 of the 3 compendia recommends a particular cancer treatment, Medicare is obligated to pay for it.
Conflicts of interest related to ties between the compendia developers and the pharmaceutical industry are being examined in a report to be delivered to Medicare soon. Some experts contributing to the National Comprehensive Cancer Network (NCCN) guidelines, which Medicare uses, have ties to drug companies. Similarly, the American Hospital Formulary compendium, which Medicare consults, used to be published by a nonprofit group. It now operates through the Foundation for Evidence-Based Medicine, to which a drug company can pay a $50,000 fee to have its drug reviewed for inclusion in the compendium. Less than one-third of such reviews result in a recommendation, however.
Medicare officials acknowledge that various issues that still need to be resolved, but they stand behind their choice of compendia.
Jimmy B
April 14, 2008 Pfizer Anti-CTLA4 antibody trial for melanoma stopped for futility ... Jim Breitfeller's point of View
April 14, 2008 Pfizer Anti-CTLA4 antibody trial for melanoma stopped for futility.
"The Data Safety Monitoring Board halted the Phase III randomized open label trial comparing the Pfizer anti-CTLA4 antibody, tremelimumab, to "standard" (and generally ineffective) chemotherapy for metastatic melanoma. The Board has reported that there is no statistical difference between the primary endpoint, overall survival, between the two study arms. Further, statistical analysis reportedly shows that further examination is "futile" or basically unlikely to ever show a statistical difference.
This is a major setback for the hopes of many investigators and patients who felt that the anti-CTLA4 antibodies represent an encouraging potential new therapy for metastatic melanoma, a disease for which there is no universally accepted or generally effective therapy.
Will this completely halt all efforts by Pfizer to develop this drug in melanoma?"
Authored by:
Eric Whitman, MD, is a Medical Director of the Office of Grants and Research for Atlantic Health System in Morristown, New Jersey
The A3671009 Phase III trial in 630 advanced melanoma patients was investigating tremelimumab compared to standard chemotherapy, consisting of dacarbazine and Schering-Plough's Temodar (temozolomide).
I for one, Believe it works but it must be in combination with Interluekin-2. See the Phase III trial was a administered as a single agent.
With my first hand experience, I did one cycle of CTLA-4 therapy and then switched to Interluekin-2. Base on the immune system pathway, the CTLA-4 blockage only activated one signal by attaching to the B7 receptor. It is all in the Medical literature. To get the Immune system to respond, it needs second signal (cell to cell). That is done with IL-2. It is my belief, that there must be time in between therapies to set the pathway and the microenvironment in motion.
I hope Pfizer doesn't give up on this therapy.
Jimmy B
"The Data Safety Monitoring Board halted the Phase III randomized open label trial comparing the Pfizer anti-CTLA4 antibody, tremelimumab, to "standard" (and generally ineffective) chemotherapy for metastatic melanoma. The Board has reported that there is no statistical difference between the primary endpoint, overall survival, between the two study arms. Further, statistical analysis reportedly shows that further examination is "futile" or basically unlikely to ever show a statistical difference.
This is a major setback for the hopes of many investigators and patients who felt that the anti-CTLA4 antibodies represent an encouraging potential new therapy for metastatic melanoma, a disease for which there is no universally accepted or generally effective therapy.
Will this completely halt all efforts by Pfizer to develop this drug in melanoma?"
Authored by:
Eric Whitman, MD, is a Medical Director of the Office of Grants and Research for Atlantic Health System in Morristown, New Jersey
The A3671009 Phase III trial in 630 advanced melanoma patients was investigating tremelimumab compared to standard chemotherapy, consisting of dacarbazine and Schering-Plough's Temodar (temozolomide).
I for one, Believe it works but it must be in combination with Interluekin-2. See the Phase III trial was a administered as a single agent.
With my first hand experience, I did one cycle of CTLA-4 therapy and then switched to Interluekin-2. Base on the immune system pathway, the CTLA-4 blockage only activated one signal by attaching to the B7 receptor. It is all in the Medical literature. To get the Immune system to respond, it needs second signal (cell to cell). That is done with IL-2. It is my belief, that there must be time in between therapies to set the pathway and the microenvironment in motion.
I hope Pfizer doesn't give up on this therapy.
Jimmy B
Follow Up of Dr. Flaherty’s Conference Call on Targeted Therapy Melanoma..Jim Breitfeller
As we all know Melanoma is on the rise in the United States and 65000 will be diagnosed this year alone. 8400 will die of the disease. Despite 30 years of research in Melanoma, we only have two FDA approved drugs at the present for Metastatic Melanoma. One is Dicarbazine also known as DACARBAZINE... NSC-45388, TOSLAB 25071, DICARBAZINE, DACARBAZINE RELATED COMPOUND A 5- AMINOIMIDAZOLE-4-CARBOXAMIDE HCL USP(CRM STANDARD), DACARBAZINE USP STANDARD, (DTIC).
It is usually administered as a single agent and has a clinical response of 5 to 10 percent. Part of the major problem is that the Melanoma Cells can become chemical resistant to the therapy because the cells can morph based on their microenvironment.1
The United States Food and Drug Administration (FDA) officially recognized the efficacy of Interluekin -2 (IL-2) in patients with metastatic melanoma by granting approval for commercial marketing of the drug for that indication in 1998, based on tumor response and survival data generated from clinical trials of the intermittent high-dose bolus regimen.
Today, we are in a new era of therapy. Immunotherapy has come to the forefront of Melanoma Therapy. Researchers are beginning to crack the code and are making great strides inmmunotherapy.
To be able to unravel the “Melanoma Maze” as I call it, we need to know “How Melanoma works.” In this next section, It may get very detailed, but I will try to explain with the help of the experts and a dictionary. I am learning like you so please bear with me.
So here it goes!!!!!
Cancer begins in cells, the building blocks that make up tissues. Tissues make up the organs of the body. Normally, cells grow and divide to form new cells as the body needs them. When cells grow old, they die, and new cells take their place.
Sometimes this orderly process goes wrong and new cells form when the body does not need them, and old cells do not die when they should. These extra cells can form a mass of tissue called a growth or tumor. Not all tumors are cancer.
In Melanoma, the cause is by the uncontrolled, unregulated growth of Melanocytes. The cell cycle proceeds unregulated, and cell growth proliferates.
It has been suggested that there are Five Stages to Metastatic Melanoma .2
Five stages of tumor progression have been suggested:
1) Benign melanocytic nevi- Melanocytic nevi are benign neoplasms or hamartomas composed of melanocytes, the pigment-producing cells that constitutively colonize the epidermis. The malignant analogue of a melanocytic nevus is melanoma. This where Melanoma can start, but can also start at eyes, ears, GI tract, leptomeninges, and oral and genital mucous membranes and even sometimes the primary site is unknown and is called “Occult Primary Melanoma”.
2) Melanocytic nevi with architectural and cytologic atypia (dysplastic nevi) “An atypical nevus, usually larger than 5 millimeters in diameter with variable pigmentation and ill-defined borders, marked by melanocytic dysplasia and associated with an increased risk for the development of nonfamilial cutaneous malignant melanoma. Also called dysplastic nevus3” The Precursor to melanoma.
“The sequence of events in which normal melanocytes transform into melanoma cells, referred to as melanomagenesis, is poorly understood. It likely involves a multistep process of progressive genetic mutations that (1) alter cell proliferation, differentiation, and death and (2) impact susceptibility to the carcinogenic effects of ultraviolet radiation.4”
3) Primary malignant melanoma, radial growth phase
This is early pattern of growth of cutaneous malignant melanoma in which tumor cells spread laterally into the epidermis. This is where if it is identified and removed with surgery, there is 95 % cure rate. The Melanoma Cells have not entered into the lymphatic system.
4) Primary malignant melanoma, vertical growth phase
The late pattern of growth of cutaneous malignant melanoma in which tumor cells spread from the epidermis into the dermis.

Figure 1. Human Skin5
5) Metastatic malignant melanoma
Each step in tumorigenesis is marked by a new clone of cells with growth advantages over the surrounding tissues. The cancer, as it invades in its place of origin, may also work its way into blood vessels. If this occurs, it provides yet another route for the cancer to spread to other organs of the body. When the cancer spreads elsewhere in the body, it has become systemic in extent and the tumor growing elsewhere is known as a metastasis.
What are the different Types of Malignant Melanoma?
1) Superficial spreading melanoma
2) Nodular melanoma
3) Lentigo maligna melanoma
4) Acral melanoma.
5) Ocular Melanoma
Superficial spreading melanoma does just as it sounds. It is on the surface of your skin and spreads horizontally. It is the most common and is a bout 65% of the Melanoma reported cases.
Nodular melanoma is a much less common form of melanoma. It usually starts as a raised lesion that is dark black-blue or bluish-red, however some can lack color. Nodular melanomas account for approximately 15% of cases. It is fast spreading and usually grows vertically in both directions.
Lentigo maligna melanoma is usually found on the palms of the hands, soles of the feet and/or around the toenails. 5-15% of all cases
Acral melanoma is an uncommon type of melanoma. It is the most common type seen in nonwhite individuals. It usually occurs on the palms and soles. Sometimes it occurs on the vulva and vagina. Acral melanoma accounts for 5% of Melanoma cases.
Ocular melanoma occurs in the eye and is estimated that 2% of the total melanoma diagnosed per year is ocular.
So where do we go from here?
Since we now know that the cause for this unregulated growth of Melanocytes is mutation in certain genes, we need to know the signaling pathways that cells took to grow. So researchers like Dr. Smalley, Dr Herlyn and many others have recently identified that the activating mutation in the BRAF gene on chromosome 7 linked to 60% of the cases in Melanoma.6
“This gene encodes a protein belonging to the raf/mil family of serine/threonine protein kinases. This protein plays a role in regulating the MAP kinase/ERKs signaling pathway, which affects cell division, differentiation, and secretion.
Mutations in this gene are associated with cardiofaciocutaneous syndrome, a disease characterized by heart defects, mental retardation and a distinctive facial appearance. Mutations in this gene have also been associated with various cancers, including non-Hodgkin lymphoma, colorectal cancer, malignant melanoma, thyroid carcinoma, non-small cell lung carcinoma, and adenocarcinoma of lung.”7
Our understanding of B-RAF regulation was greatly increased recently when the crystal structure of the B-RAF kinase domain bound to the small molecule inhibitor BAY43-9006 was solved (Wan et al., 2004).
So in 2006, in Cancer Research 66, 1611-1619, February 1, 2006], A research paper came out entitled: The Raf Inhibitor BAY 43-9006 (Sorafenib) Induces Caspase-Independent Apoptosis in Melanoma Cells. David J. Panka1, Wei Wang2, Michael B. Atkins1 and James W. Mier1
1 Division of Oncology, Beth Israel Deaconess Medical Center and Harvard Medical School and 2 Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston, Massachusetts
The paper caused excitement among researchers in that Raf Inhibitor was a way to block the pathway and cause apoptosis in Melanoma. This is a form of cell death in which a programmed sequence of events leads to the elimination of cells without releasing harmful substances into the surrounding area.
So what are the signaling pathways known to date?
Figure 2. Signaling pathways affected by the common primary changes in human melanoma, and some inter-relationships8
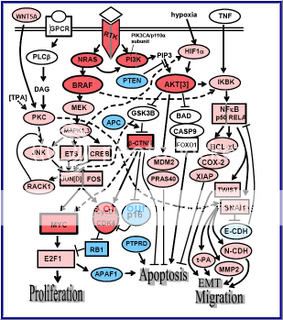
“Molecules shown in bright red are those clonally amplified or activated in some melanomas; those in bright blue are clonally deleted or inactivated in some melanomas. Pale red and pale blue indicate proteins that are at least secondarily upregulated or downregulated in melanoma, respectively. Rectangles indicate transcription factors. Some components have a larger outline; this is just to emphasize apparently important signaling nodes, and does not reflect molecular size.”
Attempts have been made to focus on pathways known to be present and active in melanoma cells (Dr. Smalley, Dr. Herlyn), although some sections of pathways are derived from the broader literature, and with the help of the pathway maps of Weinberg.9
Figure 3. Schemic of known active signaling pathways in Melanoma
by Dr. Smalley and Dr. Herlyn6
As you can see, from the diagrams, some of these pathways are intertwined and make the research more difficult but not impossible. Careful planning of the experimentations may yield new target therapies down the road. There may be a combo of Chemo and Immunology together to stabilize the Melanoma Beast.
So Now, Where does the Patients come into play?
We need to start by having our Blood typed for Biomarker. A biological marker, or biomarker, is a phenotypic parameter (eg, a substance, structure, or process) that is measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. This step One. Step two is the biopsy of your tumor and getting it typed/mapped. If the Oncologist can get the pathway that is mutated, They will be able to direct to the right therapy base on that mutation.
Example: Kit Mutation
“THURSDAY, April 17 2008 (HealthDay News) -- The cancer drug Gleevec has forced metastatic melanoma into remission for the first time, report researchers at the Dana-Farber Cancer Institute in Boston.
The case involves a 79-year-old woman with melanoma tumors in several parts of her abdomen. The tumor cells carried an abnormality in a gene called KIT, so the patient was enrolled in a clinical trail of the drug imatinib (Gleevec), which targets the KIT gene.
Four weeks after the woman started therapy, there was dramatic reduction in tumor size and metabolism. Two of the tumor masses had vanished, and several others were much smaller. After four months, the tumors were still in check and, nine months later, the women was still taking the drug and her condition remained stable.
The report was published in the April 20 issue of the Journal of Clinical Oncology .
"This is the first proof of principle that we can find an Achilles' heel in melanoma and by targeting that gene with a drug, cause the [tumor cells] to die. It is especially exciting because there haven't been any effective treatments for melanoma patients with metastatic disease," study author Dr. Stephen Hodi said in a prepared statement.
He said this case may involve just one patient, but should inspire new hope in the fight against melanoma. Because previous research failed to identify any genetic weak point that could be targeted to stop melanoma cell growth, some researchers believed that no such Achilles' heel existed for melanoma cells. The discovery of this one suggests there may be others.”
SOURCE: Dana-Farber Cancer Institute, news release, April 17, 2008
I believe we are now able to use the molecular profile of disease to deliver the right therapy to the right patient at the right time. But we must first build the infrastructure that will support Targeted Therapy with Personalized Medicine. This will require a Novel approach to the Global Healthcare seen. We would need a seamless communication along with various components including a national tissue repository, molecular diagnostic standards and procedure in place. A network of clinical sites working in collaboration to identify cohorts of patients with similar abnormal molecular profiles that is in a database and is available to Oncologists and Medical Researchers alike, a bioinformatic infrastructure integrating all of these elements.
It may be already here!!!!!!
Big Changes at BiobankCentral
Posted: 27 Jan 2009 10:36 AM CST
by Kate Blenner, Program Analyst, FasterCures10
We at FasterCures are very pleased to announce some big changes to BiobankCentral.org, the Web site we have established to highlight the importance of biobanks to medical research. This site links researchers to resources, encourages the donation of specimens, and educates the public about the benefits of research on banked biospecimens. After interviewing key stakeholders, including patient advocates, biobank operators, and leaders in the field of biospecimen research, we will begin staging some new features and functions that will make Biobankcentral.org even more useful to visitors hoping to learn more about these critical resources.
The first of these new features is the Spotlight on Innovation which will highlight individuals and organizations doing exceptionally innovative work in biobanking. Our first Spotlight focuses on the Susan G. Komen for the Cure® Tissue Bank at the Indiana University Simon Cancer Center, or Komen Tissue Bank (KTB) for short. This bank’s mission is to collect samples of normal, healthy breast tissue and other biospecimens from healthy women for breast cancer research. Yes, you read that correctly—normal tissue. Healthy women.
In its 1998 priorities for cancer research, the National Cancer Institute identified the lack of knowledge about the normal biology and development of the mammary gland as a significant barrier to finding a breast cancer cure. Most research to date has focused on characterizing diseased tissue, but without the frame of reference of how healthy tissue develops and functions opportunities for a cure could be missed. Complicating the issue was a shortage of normal tissue available for study. The NCI’s recommendations to address the ‘tissue issue’ languished for a few years, until some motivated advocates and clinicians at IU Simon Cancer Center decided to form the KTB.
Despite initial skepticism that healthy women would want to go through an invasive collection procedure, KTB put its faith in the motivation of the breast cancer advocacy community—and it paid off. They have collected thousands of samples to date, and communities across the country have asked KTB to set up its collection tent at their local Race for the Cure events. As bank co-founder and patient advocate Connie Rufenbarger told me: “These women have walked, they’ve written checks, they’ve lit candles—they’ve done everything they can to demonstrate they want to help. [The response] really speaks to the fact that there isn’t a whole lot you could ask that women wouldn’t give you to cure this disease.”
I hope you enjoy this first Spotlight of the Komen Tissue Bank as much as I enjoyed speaking with its remarkable founders and staff. If you have a moment, stop by the KTB Web site to find out how you can get involved in their work to find a cure. And, of course, keep an eye on BiobankCentral.org—we have many more exciting new changes to come.
References
1. Cedric Gaggioli and Erik Sahai- Melanoma invasion – current knowledge and future directions Pigment Cell Res. 2007 20; 161–172
2. http://www.clevelandclinicmeded.com/medicalpubs/
3. http://www.answers.com/topic/malignant-melanoma
4. Demierre MF, Nathanson L. Chemoprevention of melanoma: an unexplored strategy. J Clin Oncol. Jan 1 2003;21(1):158-65. [Medline]
5. http://www.mydr.com.au/files/images/categories/skinhair/skinstructure.gif
6. Keiran S.M. Smalley and Meenhard Herlyn Targeting Intracellular Signaling Pathways as a Novel Strategy in Melanoma Therapeutics Ann. N.Y. Acad. Sci. 1059: 1–10 (2005). 2005 New York Academy of Sciences.doi: 10.1196/annals.1339.005
7. http://www.genecards.org/cgi-bin/carddisp.pl?gene=BRAF
8. Dorothy C Bennett- How to make a melanoma: what do we know of the primary clonal events? Division of Basic Medical Sciences, St George's, University of London, Cranmer Terrace, London SW17 0RE, UK.
9. R. A. Weinberg The Biology of Cancer Garland Science, Taylor & Francis Group,. LLC, London, 2007, 864 pp., ISBN-10 0-8153-4078-8/
10. http://www.fastercures.org/
It is usually administered as a single agent and has a clinical response of 5 to 10 percent. Part of the major problem is that the Melanoma Cells can become chemical resistant to the therapy because the cells can morph based on their microenvironment.1
The United States Food and Drug Administration (FDA) officially recognized the efficacy of Interluekin -2 (IL-2) in patients with metastatic melanoma by granting approval for commercial marketing of the drug for that indication in 1998, based on tumor response and survival data generated from clinical trials of the intermittent high-dose bolus regimen.
Today, we are in a new era of therapy. Immunotherapy has come to the forefront of Melanoma Therapy. Researchers are beginning to crack the code and are making great strides inmmunotherapy.
To be able to unravel the “Melanoma Maze” as I call it, we need to know “How Melanoma works.” In this next section, It may get very detailed, but I will try to explain with the help of the experts and a dictionary. I am learning like you so please bear with me.
So here it goes!!!!!
Cancer begins in cells, the building blocks that make up tissues. Tissues make up the organs of the body. Normally, cells grow and divide to form new cells as the body needs them. When cells grow old, they die, and new cells take their place.
Sometimes this orderly process goes wrong and new cells form when the body does not need them, and old cells do not die when they should. These extra cells can form a mass of tissue called a growth or tumor. Not all tumors are cancer.
In Melanoma, the cause is by the uncontrolled, unregulated growth of Melanocytes. The cell cycle proceeds unregulated, and cell growth proliferates.
It has been suggested that there are Five Stages to Metastatic Melanoma .2
Five stages of tumor progression have been suggested:
1) Benign melanocytic nevi- Melanocytic nevi are benign neoplasms or hamartomas composed of melanocytes, the pigment-producing cells that constitutively colonize the epidermis. The malignant analogue of a melanocytic nevus is melanoma. This where Melanoma can start, but can also start at eyes, ears, GI tract, leptomeninges, and oral and genital mucous membranes and even sometimes the primary site is unknown and is called “Occult Primary Melanoma”.
2) Melanocytic nevi with architectural and cytologic atypia (dysplastic nevi) “An atypical nevus, usually larger than 5 millimeters in diameter with variable pigmentation and ill-defined borders, marked by melanocytic dysplasia and associated with an increased risk for the development of nonfamilial cutaneous malignant melanoma. Also called dysplastic nevus3” The Precursor to melanoma.
“The sequence of events in which normal melanocytes transform into melanoma cells, referred to as melanomagenesis, is poorly understood. It likely involves a multistep process of progressive genetic mutations that (1) alter cell proliferation, differentiation, and death and (2) impact susceptibility to the carcinogenic effects of ultraviolet radiation.4”
3) Primary malignant melanoma, radial growth phase
This is early pattern of growth of cutaneous malignant melanoma in which tumor cells spread laterally into the epidermis. This is where if it is identified and removed with surgery, there is 95 % cure rate. The Melanoma Cells have not entered into the lymphatic system.
4) Primary malignant melanoma, vertical growth phase
The late pattern of growth of cutaneous malignant melanoma in which tumor cells spread from the epidermis into the dermis.

Figure 1. Human Skin5
5) Metastatic malignant melanoma
Each step in tumorigenesis is marked by a new clone of cells with growth advantages over the surrounding tissues. The cancer, as it invades in its place of origin, may also work its way into blood vessels. If this occurs, it provides yet another route for the cancer to spread to other organs of the body. When the cancer spreads elsewhere in the body, it has become systemic in extent and the tumor growing elsewhere is known as a metastasis.
What are the different Types of Malignant Melanoma?
1) Superficial spreading melanoma
2) Nodular melanoma
3) Lentigo maligna melanoma
4) Acral melanoma.
5) Ocular Melanoma
Superficial spreading melanoma does just as it sounds. It is on the surface of your skin and spreads horizontally. It is the most common and is a bout 65% of the Melanoma reported cases.
Nodular melanoma is a much less common form of melanoma. It usually starts as a raised lesion that is dark black-blue or bluish-red, however some can lack color. Nodular melanomas account for approximately 15% of cases. It is fast spreading and usually grows vertically in both directions.
Lentigo maligna melanoma is usually found on the palms of the hands, soles of the feet and/or around the toenails. 5-15% of all cases
Acral melanoma is an uncommon type of melanoma. It is the most common type seen in nonwhite individuals. It usually occurs on the palms and soles. Sometimes it occurs on the vulva and vagina. Acral melanoma accounts for 5% of Melanoma cases.
Ocular melanoma occurs in the eye and is estimated that 2% of the total melanoma diagnosed per year is ocular.
So where do we go from here?
Since we now know that the cause for this unregulated growth of Melanocytes is mutation in certain genes, we need to know the signaling pathways that cells took to grow. So researchers like Dr. Smalley, Dr Herlyn and many others have recently identified that the activating mutation in the BRAF gene on chromosome 7 linked to 60% of the cases in Melanoma.6
“This gene encodes a protein belonging to the raf/mil family of serine/threonine protein kinases. This protein plays a role in regulating the MAP kinase/ERKs signaling pathway, which affects cell division, differentiation, and secretion.
Mutations in this gene are associated with cardiofaciocutaneous syndrome, a disease characterized by heart defects, mental retardation and a distinctive facial appearance. Mutations in this gene have also been associated with various cancers, including non-Hodgkin lymphoma, colorectal cancer, malignant melanoma, thyroid carcinoma, non-small cell lung carcinoma, and adenocarcinoma of lung.”7
Our understanding of B-RAF regulation was greatly increased recently when the crystal structure of the B-RAF kinase domain bound to the small molecule inhibitor BAY43-9006 was solved (Wan et al., 2004).
So in 2006, in Cancer Research 66, 1611-1619, February 1, 2006], A research paper came out entitled: The Raf Inhibitor BAY 43-9006 (Sorafenib) Induces Caspase-Independent Apoptosis in Melanoma Cells. David J. Panka1, Wei Wang2, Michael B. Atkins1 and James W. Mier1
1 Division of Oncology, Beth Israel Deaconess Medical Center and Harvard Medical School and 2 Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston, Massachusetts
The paper caused excitement among researchers in that Raf Inhibitor was a way to block the pathway and cause apoptosis in Melanoma. This is a form of cell death in which a programmed sequence of events leads to the elimination of cells without releasing harmful substances into the surrounding area.
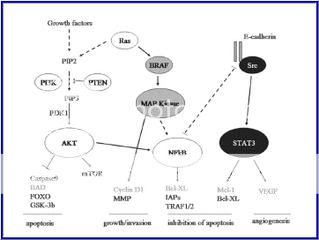
So what are the signaling pathways known to date?
Figure 2. Signaling pathways affected by the common primary changes in human melanoma, and some inter-relationships8

“Molecules shown in bright red are those clonally amplified or activated in some melanomas; those in bright blue are clonally deleted or inactivated in some melanomas. Pale red and pale blue indicate proteins that are at least secondarily upregulated or downregulated in melanoma, respectively. Rectangles indicate transcription factors. Some components have a larger outline; this is just to emphasize apparently important signaling nodes, and does not reflect molecular size.”
Attempts have been made to focus on pathways known to be present and active in melanoma cells (Dr. Smalley, Dr. Herlyn), although some sections of pathways are derived from the broader literature, and with the help of the pathway maps of Weinberg.9
Figure 3. Schemic of known active signaling pathways in Melanoma
by Dr. Smalley and Dr. Herlyn6

As you can see, from the diagrams, some of these pathways are intertwined and make the research more difficult but not impossible. Careful planning of the experimentations may yield new target therapies down the road. There may be a combo of Chemo and Immunology together to stabilize the Melanoma Beast.
So Now, Where does the Patients come into play?
We need to start by having our Blood typed for Biomarker. A biological marker, or biomarker, is a phenotypic parameter (eg, a substance, structure, or process) that is measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. This step One. Step two is the biopsy of your tumor and getting it typed/mapped. If the Oncologist can get the pathway that is mutated, They will be able to direct to the right therapy base on that mutation.
Example: Kit Mutation
“THURSDAY, April 17 2008 (HealthDay News) -- The cancer drug Gleevec has forced metastatic melanoma into remission for the first time, report researchers at the Dana-Farber Cancer Institute in Boston.
The case involves a 79-year-old woman with melanoma tumors in several parts of her abdomen. The tumor cells carried an abnormality in a gene called KIT, so the patient was enrolled in a clinical trail of the drug imatinib (Gleevec), which targets the KIT gene.
Four weeks after the woman started therapy, there was dramatic reduction in tumor size and metabolism. Two of the tumor masses had vanished, and several others were much smaller. After four months, the tumors were still in check and, nine months later, the women was still taking the drug and her condition remained stable.
The report was published in the April 20 issue of the Journal of Clinical Oncology .
"This is the first proof of principle that we can find an Achilles' heel in melanoma and by targeting that gene with a drug, cause the [tumor cells] to die. It is especially exciting because there haven't been any effective treatments for melanoma patients with metastatic disease," study author Dr. Stephen Hodi said in a prepared statement.
He said this case may involve just one patient, but should inspire new hope in the fight against melanoma. Because previous research failed to identify any genetic weak point that could be targeted to stop melanoma cell growth, some researchers believed that no such Achilles' heel existed for melanoma cells. The discovery of this one suggests there may be others.”
SOURCE: Dana-Farber Cancer Institute, news release, April 17, 2008
I believe we are now able to use the molecular profile of disease to deliver the right therapy to the right patient at the right time. But we must first build the infrastructure that will support Targeted Therapy with Personalized Medicine. This will require a Novel approach to the Global Healthcare seen. We would need a seamless communication along with various components including a national tissue repository, molecular diagnostic standards and procedure in place. A network of clinical sites working in collaboration to identify cohorts of patients with similar abnormal molecular profiles that is in a database and is available to Oncologists and Medical Researchers alike, a bioinformatic infrastructure integrating all of these elements.
It may be already here!!!!!!
Big Changes at BiobankCentral
Posted: 27 Jan 2009 10:36 AM CST
by Kate Blenner, Program Analyst, FasterCures10
We at FasterCures are very pleased to announce some big changes to BiobankCentral.org, the Web site we have established to highlight the importance of biobanks to medical research. This site links researchers to resources, encourages the donation of specimens, and educates the public about the benefits of research on banked biospecimens. After interviewing key stakeholders, including patient advocates, biobank operators, and leaders in the field of biospecimen research, we will begin staging some new features and functions that will make Biobankcentral.org even more useful to visitors hoping to learn more about these critical resources.
The first of these new features is the Spotlight on Innovation which will highlight individuals and organizations doing exceptionally innovative work in biobanking. Our first Spotlight focuses on the Susan G. Komen for the Cure® Tissue Bank at the Indiana University Simon Cancer Center, or Komen Tissue Bank (KTB) for short. This bank’s mission is to collect samples of normal, healthy breast tissue and other biospecimens from healthy women for breast cancer research. Yes, you read that correctly—normal tissue. Healthy women.
In its 1998 priorities for cancer research, the National Cancer Institute identified the lack of knowledge about the normal biology and development of the mammary gland as a significant barrier to finding a breast cancer cure. Most research to date has focused on characterizing diseased tissue, but without the frame of reference of how healthy tissue develops and functions opportunities for a cure could be missed. Complicating the issue was a shortage of normal tissue available for study. The NCI’s recommendations to address the ‘tissue issue’ languished for a few years, until some motivated advocates and clinicians at IU Simon Cancer Center decided to form the KTB.
Despite initial skepticism that healthy women would want to go through an invasive collection procedure, KTB put its faith in the motivation of the breast cancer advocacy community—and it paid off. They have collected thousands of samples to date, and communities across the country have asked KTB to set up its collection tent at their local Race for the Cure events. As bank co-founder and patient advocate Connie Rufenbarger told me: “These women have walked, they’ve written checks, they’ve lit candles—they’ve done everything they can to demonstrate they want to help. [The response] really speaks to the fact that there isn’t a whole lot you could ask that women wouldn’t give you to cure this disease.”
I hope you enjoy this first Spotlight of the Komen Tissue Bank as much as I enjoyed speaking with its remarkable founders and staff. If you have a moment, stop by the KTB Web site to find out how you can get involved in their work to find a cure. And, of course, keep an eye on BiobankCentral.org—we have many more exciting new changes to come.
References
1. Cedric Gaggioli and Erik Sahai- Melanoma invasion – current knowledge and future directions Pigment Cell Res. 2007 20; 161–172
2. http://www.clevelandclinicmeded.com/medicalpubs/
3. http://www.answers.com/topic/malignant-melanoma
4. Demierre MF, Nathanson L. Chemoprevention of melanoma: an unexplored strategy. J Clin Oncol. Jan 1 2003;21(1):158-65. [Medline]
5. http://www.mydr.com.au/files/images/categories/skinhair/skinstructure.gif
6. Keiran S.M. Smalley and Meenhard Herlyn Targeting Intracellular Signaling Pathways as a Novel Strategy in Melanoma Therapeutics Ann. N.Y. Acad. Sci. 1059: 1–10 (2005). 2005 New York Academy of Sciences.doi: 10.1196/annals.1339.005
7. http://www.genecards.org/cgi-bin/carddisp.pl?gene=BRAF
8. Dorothy C Bennett- How to make a melanoma: what do we know of the primary clonal events? Division of Basic Medical Sciences, St George's, University of London, Cranmer Terrace, London SW17 0RE, UK.
9. R. A. Weinberg The Biology of Cancer Garland Science, Taylor & Francis Group,. LLC, London, 2007, 864 pp., ISBN-10 0-8153-4078-8/
10. http://www.fastercures.org/
Tuesday, January 27, 2009
Targeting Immune Stimulation: Clinical Application of Immunology Melanoma ..Jim Breitfeller
Contributing Writers: F. Stephen Hodi, MD and Lauren Cerruto
Contributing Editor: F. Stephen Hodi, MD
Editor-in-Chief: Jeffrey S. Weber, MD, PhD
Efforts to develop immunotherapies for cancer have historically involved vaccines and individual cytokines, which have produced benefits to only a small percentage of patients.
Recently, improved understanding of immune processes, such as the role of T-cell costimulatory molecules and regulatory molecules, has led to renewed efforts to develop immunotherapies to treat cancer. Current clinical trials are utilizing monoclonal antibodies (mAbs) to modulate costimulatory effects in the treatment of a variety of cancers. For example, two fully humanized mAbs that block cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) have advanced to phase III clinical studies in metastatic melanoma. Antitumor activity has been observed in melanoma and other malignancies after treatment with anti-CTLA-4 antibodies, as well as the potential for autoimmune-related toxicities.
Rationale for Targeting CTLA-4 in Patients with Cancer
http://www.livingmedicaltextbook.org/Activity/index.cfm?showfile=b&jn=1843&sj=1843.04
Living Medical Textbook Targeting Immune Stimulation: Clinical Application of Immunology
Jimmy B
Contributing Editor: F. Stephen Hodi, MD
Editor-in-Chief: Jeffrey S. Weber, MD, PhD
Efforts to develop immunotherapies for cancer have historically involved vaccines and individual cytokines, which have produced benefits to only a small percentage of patients.
Recently, improved understanding of immune processes, such as the role of T-cell costimulatory molecules and regulatory molecules, has led to renewed efforts to develop immunotherapies to treat cancer. Current clinical trials are utilizing monoclonal antibodies (mAbs) to modulate costimulatory effects in the treatment of a variety of cancers. For example, two fully humanized mAbs that block cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) have advanced to phase III clinical studies in metastatic melanoma. Antitumor activity has been observed in melanoma and other malignancies after treatment with anti-CTLA-4 antibodies, as well as the potential for autoimmune-related toxicities.
Rationale for Targeting CTLA-4 in Patients with Cancer
http://www.livingmedicaltextbook.org/Activity/index.cfm?showfile=b&jn=1843&sj=1843.04
Living Medical Textbook Targeting Immune Stimulation: Clinical Application of Immunology
Jimmy B
Labels:
CTLA-4,
Dr. Hodi,
Dr. Weber,
Melanoma,
Melanoma Lectures
Latest survival data from three Phase II ipilimumab studies!!!!! Melanoma ..Jim Breitfeller
Latest survival data from three Phase II ipilimumab studies showed almost half of previously treated metastatic melanoma patients alive beyond one year1,2,3
- Data presented at the 33rd Congress of the European Society for Medical Oncology -
Stockholm – 16 September 2008 – Bristol-Myers Squibb announced updated survival data from three Phase II studies of ipilimumab in patients with advanced metastatic melanoma (Stage III or IV), which showed that approximately half of previously-treated patients who received ipilimumab (10 mg/kg) remained alive beyond one year. 1,2,3 Ipilimumab is designed to block the activity of CTLA-4 (a molecule on T-cells that plays a critical role in regulating natural immune responses), and thereby activates the immune system to fight metastatic melanoma.4,5
The results are based on a follow-up of the patient population from studies 008, 022 and 007. 47 – 51 percent of patients with advanced metastatic melanoma treated with 10 mg/kg of ipilimumab (induction and maintenance) showed a consistent survival rate of one-year.1,2,3 Specifically, the results show:
* 47 percent of patients who had progressed while on or after receiving standard treatment achieved one year survival (Study 008)1
* 48 percent of patients who were previously treated, relapsed or failed to respond to experimental treatment or were unable to tolerate currently approved therapies achieved one-year survival (Study 022)2
* 51 percent of patients previously treated with therapy other than ipilimumab achieved one year survival (Study 007)3
Recent medical literature, based on a meta-analysis of 42 Phase II trials with 2,100 patients, reported a one-year survival rate of approximately 25.5 percent for patients with Stage III or IV metastatic melanoma, the most advanced type of the disease.6
http://www.countrydoctor.co.uk/education/Education%20-%20Metastatic%20melanoma%20hopes.htm
Jimmy B
- Data presented at the 33rd Congress of the European Society for Medical Oncology -
Stockholm – 16 September 2008 – Bristol-Myers Squibb announced updated survival data from three Phase II studies of ipilimumab in patients with advanced metastatic melanoma (Stage III or IV), which showed that approximately half of previously-treated patients who received ipilimumab (10 mg/kg) remained alive beyond one year. 1,2,3 Ipilimumab is designed to block the activity of CTLA-4 (a molecule on T-cells that plays a critical role in regulating natural immune responses), and thereby activates the immune system to fight metastatic melanoma.4,5
The results are based on a follow-up of the patient population from studies 008, 022 and 007. 47 – 51 percent of patients with advanced metastatic melanoma treated with 10 mg/kg of ipilimumab (induction and maintenance) showed a consistent survival rate of one-year.1,2,3 Specifically, the results show:
* 47 percent of patients who had progressed while on or after receiving standard treatment achieved one year survival (Study 008)1
* 48 percent of patients who were previously treated, relapsed or failed to respond to experimental treatment or were unable to tolerate currently approved therapies achieved one-year survival (Study 022)2
* 51 percent of patients previously treated with therapy other than ipilimumab achieved one year survival (Study 007)3
Recent medical literature, based on a meta-analysis of 42 Phase II trials with 2,100 patients, reported a one-year survival rate of approximately 25.5 percent for patients with Stage III or IV metastatic melanoma, the most advanced type of the disease.6
http://www.countrydoctor.co.uk/education/Education%20-%20Metastatic%20melanoma%20hopes.htm
Jimmy B
Monday, January 26, 2009
Thursday, January 22, 2009
Jimmy B's Update. Melanoma .. Jim Breitfeller
You know from my messages, and I have told some people that I been researching Rose Bengal. The therapy originated downunder in Australia. It has to do with lesions.
Well,I have one that has not cleared up since 2006. The PET Scan shows no activity but I am question why it won't heal. It bleeds than scabs up and the scab falls off and bleeds again. It has been getting smaller.
Anyway I have been watching the information on Rose Bengal for over a couple of years. Well, The time has come to see if it is Melanoma
It is now in clinical trials in the States.
Phase 2 Study of Intralesional PV-10 for Metastatic Melanoma
This study is currently recruiting participants.
Verified by Provectus Pharmaceuticals, December 2008
Sponsored by: Provectus Pharmaceuticals
Information provided by: Provectus Pharmaceuticals
ClinicalTrials.gov Identifier: NCT00521053
Purpose
The primary objective of this study is to investigate the effectiveness of intralesional (IL) PV-10 for locoregional treatment of metastatic melanoma. This study will also include assessment of response in untreated bystander lesions following intralesional injection of PV-10 into targeted lesions. Additional objectives are to determine the safety profile of PV-10 following intralesional injection, and assess the pharmacokinetic profile of PV-10 in the bloodstream following intralesional injection.
Condition Intervention Phase
Melanoma
Drug: PV-10 (10% rose bengal disodium)
Phase II
I am having a needle biopsy done on February 3rd to check to see if it is
Melanoma.
Jimmy B
Well,I have one that has not cleared up since 2006. The PET Scan shows no activity but I am question why it won't heal. It bleeds than scabs up and the scab falls off and bleeds again. It has been getting smaller.
Anyway I have been watching the information on Rose Bengal for over a couple of years. Well, The time has come to see if it is Melanoma
It is now in clinical trials in the States.
Phase 2 Study of Intralesional PV-10 for Metastatic Melanoma
This study is currently recruiting participants.
Verified by Provectus Pharmaceuticals, December 2008
Sponsored by: Provectus Pharmaceuticals
Information provided by: Provectus Pharmaceuticals
ClinicalTrials.gov Identifier: NCT00521053
Purpose
The primary objective of this study is to investigate the effectiveness of intralesional (IL) PV-10 for locoregional treatment of metastatic melanoma. This study will also include assessment of response in untreated bystander lesions following intralesional injection of PV-10 into targeted lesions. Additional objectives are to determine the safety profile of PV-10 following intralesional injection, and assess the pharmacokinetic profile of PV-10 in the bloodstream following intralesional injection.
Condition Intervention Phase
Melanoma
Drug: PV-10 (10% rose bengal disodium)
Phase II
I am having a needle biopsy done on February 3rd to check to see if it is
Melanoma.
Jimmy B
Summary of the Conference Call with Dr. Flaherty on 1/21/2008 Melanoma.. Jim Breitfeller
Topic: Targeted Therapy
WHAT IS TARGETED THERAPY?
Targeted therapy is a term that refers to a drug or combination of drugs that targets a specific pathway in the growth and development of a tumor. By attacking or blocking these important targets, the therapy helps to fight the tumor itself. The targets themselves are typically various small molecules in the body that are known or suspected to induce cancer formation.
HOW ARE TARGETED THERAPIES NAMED?
The names of the major classes of targeted therapies typically include the word "anti-", or "inhibitor", together with the name of the target itself. This means that the drug blocks, (is "anti"), that particular target. Then within each class of inhibitors, there is/are the actual drug(s).
It is important to realize that a single drug can have several names, including a generic name and a brand name. This can be confusing because often the generic and brand names are used interchangeably in the literature and the media. This means you need to the names to follow the pathways.
What are the different classes of targeted therapy? In other words, what are the different targets?
There are several pathways that have been studied quite intensively and they are:
Kinase: Defintion …Any of various enzymes (proteins) that catalyze the transfer of a phosphate group from a donor, such as ADP or ATP, to an acceptor
1) Map Kinase : Mitogen-activated protein (MAP) kinases (EC 2.7.11.24) are serine/threonine-specific protein kinases that respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation, and cell survival/apoptosis
It was studied early on in the Melanoma Research.
2) PI3-Kinase: PI 3-kinases have been linked to an extraordinarily diverse group of cellular functions, including cell growth, proliferation, differentiation, motility, survival and intracellular trafficking. Many of these functions relate to the ability of class I PI 3-kinases to activate protein kinase B (PKB, aka Akt). The class IA PI 3-kinase p110α is mutated in many cancers. Many of these mutations cause the kinase to be more active. The PtdIns(3,4,5)P3 phosphatase PTEN which antagonises PI 3-kinase signalling is absent from many tumours. Hence, PI 3-kinase activity contributes significantly to cellular transformation and the development of cancer. The p110δ and p110γ isoforms regulate different aspects of immune responses. PI 3-kinases are also a key component of the insulin signaling pathway. Hence there is great interest in the role of PI 3-kinase signaling in Diabetes mellitus.).
Another pathway was the c-Kit: Receptor tyrosine kinases, such as c-Kit, are proteins whose function it is to transduce signals from the environment into the cell leading to complex behaviors such as proliferation, migration, survival and differentiation. Many of these behaviors are deregulated in cancer, which is characterized by uncontrolled proliferation, insensitivity towards death stimuli, migration of tumor cells away from the primary tumor site and in some cases also block of cellular differentiation leaving the cell in an immature proliferative state. To be able to target these processes it is vital to have a detailed understanding of the receptor function and the downstream pathways activated.
Well, they developed A c-Kit antigen:
.
Example of Targeted Therapy:
THURSDAY, April 17 2008 (HealthDay News) -- The cancer drug Gleevec has forced metastatic melanoma into remission for the first time, report researchers at the Dana-Farber Cancer Institute in Boston.
The case involves a 79-year-old woman with melanoma tumors in several parts of her abdomen. The tumor cells carried an abnormality in a gene called KIT, so the patient was enrolled in a clinical trail of the drug imatinib (Gleevec), which targets the KIT gene.
Four weeks after the woman started therapy, there was dramatic reduction in tumor size and metabolism. Two of the tumor masses had vanished, and several others were much smaller. After four months, the tumors were still in check and, nine months later, the women was still taking the drug and her condition remained stable.
The report was published in the April 20 issue of the Journal of Clinical Oncology .
"This is the first proof of principle that we can find an Achilles' heel in melanoma and by targeting that gene with a drug, cause the [tumor cells] to die. It is especially exciting because there haven't been any effective treatments for melanoma patients with metastatic disease," study author Dr. Stephen Hodi said in a prepared statement.
He said this case may involve just one patient, but should inspire new hope in the fight against melanoma. Because previous research failed to identify any genetic weak point that could be targeted to stop melanoma cell growth, some researchers believed that no such Achilles' heel existed for melanoma cells. The discovery of this one suggests there may be others.
SOURCE: Dana-Farber Cancer Institute, news release, April 17, 2008Melanoma Mutations:
Strategies for attacking melanoma may soon involve a personalized approach based on the genetic characteristics of the patient’s tumor. Recently, scientists have begun to uncover genetic mutations that drive the unchecked growth of melanoma cells. By determining the pathways that caused the mutation, they hope to create drugs that better target the specific problem.
A common genetic mutation in melanoma creates an overactive version of a protein called BRAF, which instigates cell growth and division. Other mutations activate a related protein, called N-RAS, also resulting in unchecked cell division. Drugs designed to block these overactive proteins kill melanoma cells in laboratory tests, but so far they have shown only some promise in patients. So they are starting personalize the therapy base on patient’s tumor genetics.
Near term goal is to control the progression until they can find the silver bullet. So they will use Chemo and Biological to control the Beast for now.
So, hold on to your hats and make sure that if you have a tumor that can be harvested, you should ask your oncologist to get genetic mapping/typing of the tumor. It could send you down the YELLOW BRICK ROAD to the Emerald City.
Another take away is that a cure may be based on personalized therapy. So there might not be just one cure, but many based on genetics.
Source:Oncolink and the Dictionary
Take Care
jimmy B
WHAT IS TARGETED THERAPY?
Targeted therapy is a term that refers to a drug or combination of drugs that targets a specific pathway in the growth and development of a tumor. By attacking or blocking these important targets, the therapy helps to fight the tumor itself. The targets themselves are typically various small molecules in the body that are known or suspected to induce cancer formation.
HOW ARE TARGETED THERAPIES NAMED?
The names of the major classes of targeted therapies typically include the word "anti-", or "inhibitor", together with the name of the target itself. This means that the drug blocks, (is "anti"), that particular target. Then within each class of inhibitors, there is/are the actual drug(s).
It is important to realize that a single drug can have several names, including a generic name and a brand name. This can be confusing because often the generic and brand names are used interchangeably in the literature and the media. This means you need to the names to follow the pathways.
What are the different classes of targeted therapy? In other words, what are the different targets?
There are several pathways that have been studied quite intensively and they are:
Kinase: Defintion …Any of various enzymes (proteins) that catalyze the transfer of a phosphate group from a donor, such as ADP or ATP, to an acceptor
1) Map Kinase : Mitogen-activated protein (MAP) kinases (EC 2.7.11.24) are serine/threonine-specific protein kinases that respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation, and cell survival/apoptosis
It was studied early on in the Melanoma Research.
2) PI3-Kinase: PI 3-kinases have been linked to an extraordinarily diverse group of cellular functions, including cell growth, proliferation, differentiation, motility, survival and intracellular trafficking. Many of these functions relate to the ability of class I PI 3-kinases to activate protein kinase B (PKB, aka Akt). The class IA PI 3-kinase p110α is mutated in many cancers. Many of these mutations cause the kinase to be more active. The PtdIns(3,4,5)P3 phosphatase PTEN which antagonises PI 3-kinase signalling is absent from many tumours. Hence, PI 3-kinase activity contributes significantly to cellular transformation and the development of cancer. The p110δ and p110γ isoforms regulate different aspects of immune responses. PI 3-kinases are also a key component of the insulin signaling pathway. Hence there is great interest in the role of PI 3-kinase signaling in Diabetes mellitus.).
Another pathway was the c-Kit: Receptor tyrosine kinases, such as c-Kit, are proteins whose function it is to transduce signals from the environment into the cell leading to complex behaviors such as proliferation, migration, survival and differentiation. Many of these behaviors are deregulated in cancer, which is characterized by uncontrolled proliferation, insensitivity towards death stimuli, migration of tumor cells away from the primary tumor site and in some cases also block of cellular differentiation leaving the cell in an immature proliferative state. To be able to target these processes it is vital to have a detailed understanding of the receptor function and the downstream pathways activated.
Well, they developed A c-Kit antigen:
.
Example of Targeted Therapy:
THURSDAY, April 17 2008 (HealthDay News) -- The cancer drug Gleevec has forced metastatic melanoma into remission for the first time, report researchers at the Dana-Farber Cancer Institute in Boston.
The case involves a 79-year-old woman with melanoma tumors in several parts of her abdomen. The tumor cells carried an abnormality in a gene called KIT, so the patient was enrolled in a clinical trail of the drug imatinib (Gleevec), which targets the KIT gene.
Four weeks after the woman started therapy, there was dramatic reduction in tumor size and metabolism. Two of the tumor masses had vanished, and several others were much smaller. After four months, the tumors were still in check and, nine months later, the women was still taking the drug and her condition remained stable.
The report was published in the April 20 issue of the Journal of Clinical Oncology .
"This is the first proof of principle that we can find an Achilles' heel in melanoma and by targeting that gene with a drug, cause the [tumor cells] to die. It is especially exciting because there haven't been any effective treatments for melanoma patients with metastatic disease," study author Dr. Stephen Hodi said in a prepared statement.
He said this case may involve just one patient, but should inspire new hope in the fight against melanoma. Because previous research failed to identify any genetic weak point that could be targeted to stop melanoma cell growth, some researchers believed that no such Achilles' heel existed for melanoma cells. The discovery of this one suggests there may be others.
SOURCE: Dana-Farber Cancer Institute, news release, April 17, 2008Melanoma Mutations:
Strategies for attacking melanoma may soon involve a personalized approach based on the genetic characteristics of the patient’s tumor. Recently, scientists have begun to uncover genetic mutations that drive the unchecked growth of melanoma cells. By determining the pathways that caused the mutation, they hope to create drugs that better target the specific problem.
A common genetic mutation in melanoma creates an overactive version of a protein called BRAF, which instigates cell growth and division. Other mutations activate a related protein, called N-RAS, also resulting in unchecked cell division. Drugs designed to block these overactive proteins kill melanoma cells in laboratory tests, but so far they have shown only some promise in patients. So they are starting personalize the therapy base on patient’s tumor genetics.
Near term goal is to control the progression until they can find the silver bullet. So they will use Chemo and Biological to control the Beast for now.
So, hold on to your hats and make sure that if you have a tumor that can be harvested, you should ask your oncologist to get genetic mapping/typing of the tumor. It could send you down the YELLOW BRICK ROAD to the Emerald City.
Another take away is that a cure may be based on personalized therapy. So there might not be just one cure, but many based on genetics.
Source:Oncolink and the Dictionary
Take Care
jimmy B
Sign up and the Doors of Knowlege will Open!!!! Melanoma ..Jim Breitfeller
There is a video I would like you to see, but you must sign up. It is free, and it will open doors to the medical Field.
You won't be disappointed.
Progress in the Development of Novel Therapies for Melanoma: A Video Interview With Dr. Jedd D. Wolchok
Jimmy B
You won't be disappointed.
Progress in the Development of Novel Therapies for Melanoma: A Video Interview With Dr. Jedd D. Wolchok
Jimmy B
Wednesday, January 21, 2009
Hey!!!!! Look What I Found!!!!!!! Melanoma Jim Breitfeller
While I was doing some research on Rose Bengal, I stumbled onto this website.
WWW.Curehunter.com
I am amazed at what you can find in the cyber Library.
Check out the Experts!!!!
www.curehunter.com...Melanoma
I might have to ask Moma Bear if I can spend $24.00 for a summary report.
Well it is back to the books again. HOMEWORK..HOMEWORK..HOMEWORK!!!!!
Take Care
WWW.Curehunter.com
I am amazed at what you can find in the cyber Library.
Check out the Experts!!!!
www.curehunter.com...Melanoma
I might have to ask Moma Bear if I can spend $24.00 for a summary report.
Well it is back to the books again. HOMEWORK..HOMEWORK..HOMEWORK!!!!!
Take Care
Labels:
CTLA-4,
dicarbazine,
dr. kirkwood,
Melanoma,
rose bengal,
Rosenberg
Tuesday, January 20, 2009
Phase III Trial Comparing Concurrent Biochemotherapy With Cisplatin, Vinblastine, Dacarbazine, Interleukin-2, and Interferon Alfa-2b With Cisplatin...
Phase III Trial Comparing Concurrent Biochemotherapy With Cisplatin, Vinblastine, Dacarbazine, Interleukin-2, and Interferon Alfa-2b With Cisplatin, Vinblastine, and Dacarbazine Alone in Patients With Metastatic Malignant Melanoma (E3695): A Trial Coordinated by the Eastern Cooperative Oncology Group
J Clin Oncol. 2008 Dec 10;26(35):5748-5754, MB Atkins, J Hsu, S Lee, GI Cohen, LE Flaherty, JA Sosman, VK Sondak, JM Kirkwood
The addition of immunotherapy to cisplatin and dacarbazine chemotherapy has been shown to increase response rates vs immunotherapy or chemotherapy alone in patients with metastatic melanoma. The addition of interferon alfa-2b (IFN-ą) to cisplatin, vinblastine, and dacarbazine (CVD) plus interleukin-2 (IL-2) resulted in further improvement in response rates and a marginally significant, 3-month improvement in survival time in 1 study. This combination regimen showed no survival benefit in 2 other studies, however. Promising results of studies in which immunotherapy and chemotherapy were administered concurrently, rather than sequentially, prompted the Eastern Cooperative Oncology Group (ECOG) to conduct a randomized phase III trial to evaluate a biochemotherapy (BCT) regimen of IL-2 and IFN-ą immunotherapy concurrent with CVD in 395 patients with progressive metastatic melanoma. In this ECOG trial (E3695), BCT did not improve overall survival (OS) over CVD alone and resulted in greater toxicity.
Patients randomized to the CVD arm received cisplatin, vinblastine, and dacarbazine, with antiemetics and dexamethasone. Patients randomized to the BCT arm received CVD, plus IL-2, IFN-ą, cephalexin, and granulocyte colony-stimulating factor, as well as hydration and antiemetic therapy, prophylactic acetaminophen and ranitidine, and antipruritics, antidiarrheal agents, and anxiolytics as needed. In both treatment arms, cycles were repeated at 3-week intervals for a maximum of 4 cycles. Dosage was modified in response to grade 3 or higher toxicity.
Of the 416 patients were enrolled, 395 had analyzable data, including 195 in the CVD arm and 200 in the BCT arm. There were 3 patients in the CVD arm and 5 patients in the BCT arm who did not receive their assigned therapy. Significantly fewer patients in the BCT arm than in the CVD arm received full doses of their therapies (P < .01). A total of 10 patients in the CVD arm and none in the BCT arm underwent >4 cycles of therapy.
Nearly three-quarters (73%) of patients in the CVD arm and 95% of patients in the BCT arm experienced grade 3 or higher toxicity (P = .001). The most common toxic effects included leukopenia, granulocytopenia, thrombocytopenia, anemia, infection, nausea, vomiting, hepatic and metabolic abnormalities, hypotension, and fatigue. With the exception of granulocytopenia and infection, all toxicities were significantly more common in patients who received BCT than in those who received CVD. Deaths due to treatment-related toxicity occurred in 3 patients in the CVD arm (myocardial infarction, hypotension, and infection) and 2 patients in the BCT arm (infection and renal failure).
Overall, 94% of patients died. Median OS was similar in the CVD and BCT arms (8.7 vs 9.0 months; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.8-1.17; P = .639). At 1 year, 36.9% of patients in the CVD arm and 41% in the BCT arm were alive. Median progression-free survival was significantly shorter in the CVD recipients than in the BCT recipients (2.9 vs 4.8 months; HR = .0.77; 95% CI, 0.63-0.94; P = .015). At 6 months, 25.0% and 38.9% of patients were progression free.
Response rates were similar in the CVD and BCT groups (13.8% vs 19.5%; P = .140). Complete responses occurred in 4.6% and 2.5% of patients in the CVD and BCT groups, respectively, and these responses were durable (>lasting 2 years) in 6 CVD-treated patients but only 2 BCT-treated patients. Among patients with complete or partial responses, median response duration was similar in the CVD and BCT arms (9.4 vs 6.1 months; HR = 1.47; 95% CI, 0.83-2.60; P = .181).
The E695 trial represents the largest and most definitive phase III trial of BCT ever conducted. Given that BCT improved progression-free survival but not overall survival in this trial and also was more toxic than CVD, the ECOG investigators conclude that BCT should not be considered the standard of care for patients with advanced melanoma
J Clin Oncol. 2008 Dec 10;26(35):5748-5754, MB Atkins, J Hsu, S Lee, GI Cohen, LE Flaherty, JA Sosman, VK Sondak, JM Kirkwood
The addition of immunotherapy to cisplatin and dacarbazine chemotherapy has been shown to increase response rates vs immunotherapy or chemotherapy alone in patients with metastatic melanoma. The addition of interferon alfa-2b (IFN-ą) to cisplatin, vinblastine, and dacarbazine (CVD) plus interleukin-2 (IL-2) resulted in further improvement in response rates and a marginally significant, 3-month improvement in survival time in 1 study. This combination regimen showed no survival benefit in 2 other studies, however. Promising results of studies in which immunotherapy and chemotherapy were administered concurrently, rather than sequentially, prompted the Eastern Cooperative Oncology Group (ECOG) to conduct a randomized phase III trial to evaluate a biochemotherapy (BCT) regimen of IL-2 and IFN-ą immunotherapy concurrent with CVD in 395 patients with progressive metastatic melanoma. In this ECOG trial (E3695), BCT did not improve overall survival (OS) over CVD alone and resulted in greater toxicity.
Patients randomized to the CVD arm received cisplatin, vinblastine, and dacarbazine, with antiemetics and dexamethasone. Patients randomized to the BCT arm received CVD, plus IL-2, IFN-ą, cephalexin, and granulocyte colony-stimulating factor, as well as hydration and antiemetic therapy, prophylactic acetaminophen and ranitidine, and antipruritics, antidiarrheal agents, and anxiolytics as needed. In both treatment arms, cycles were repeated at 3-week intervals for a maximum of 4 cycles. Dosage was modified in response to grade 3 or higher toxicity.
Of the 416 patients were enrolled, 395 had analyzable data, including 195 in the CVD arm and 200 in the BCT arm. There were 3 patients in the CVD arm and 5 patients in the BCT arm who did not receive their assigned therapy. Significantly fewer patients in the BCT arm than in the CVD arm received full doses of their therapies (P < .01). A total of 10 patients in the CVD arm and none in the BCT arm underwent >4 cycles of therapy.
Nearly three-quarters (73%) of patients in the CVD arm and 95% of patients in the BCT arm experienced grade 3 or higher toxicity (P = .001). The most common toxic effects included leukopenia, granulocytopenia, thrombocytopenia, anemia, infection, nausea, vomiting, hepatic and metabolic abnormalities, hypotension, and fatigue. With the exception of granulocytopenia and infection, all toxicities were significantly more common in patients who received BCT than in those who received CVD. Deaths due to treatment-related toxicity occurred in 3 patients in the CVD arm (myocardial infarction, hypotension, and infection) and 2 patients in the BCT arm (infection and renal failure).
Overall, 94% of patients died. Median OS was similar in the CVD and BCT arms (8.7 vs 9.0 months; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.8-1.17; P = .639). At 1 year, 36.9% of patients in the CVD arm and 41% in the BCT arm were alive. Median progression-free survival was significantly shorter in the CVD recipients than in the BCT recipients (2.9 vs 4.8 months; HR = .0.77; 95% CI, 0.63-0.94; P = .015). At 6 months, 25.0% and 38.9% of patients were progression free.
Response rates were similar in the CVD and BCT groups (13.8% vs 19.5%; P = .140). Complete responses occurred in 4.6% and 2.5% of patients in the CVD and BCT groups, respectively, and these responses were durable (>lasting 2 years) in 6 CVD-treated patients but only 2 BCT-treated patients. Among patients with complete or partial responses, median response duration was similar in the CVD and BCT arms (9.4 vs 6.1 months; HR = 1.47; 95% CI, 0.83-2.60; P = .181).
The E695 trial represents the largest and most definitive phase III trial of BCT ever conducted. Given that BCT improved progression-free survival but not overall survival in this trial and also was more toxic than CVD, the ECOG investigators conclude that BCT should not be considered the standard of care for patients with advanced melanoma
Labels:
Dr. Flaherty,
dr. kirkwood,
interferon,
interlukin-2,
Melanoma
Call for Patients!!!!!!! NEW CLINICAL TRIAL!!!!!!!!
Jim
We have powerful new immunotherapy treatments for patients with metastatic melanoma using cell transfer techniques (see attached publication).
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Steven A. Rosenberg M.D., Ph.D.
Chief, Surgery Branch
National Cancer Institute
10 Center Drive MSC 1201
CRC Room 3-3940
Bethesda, MD 20892
301-496-4164
sar@nih.gov
Adoptive Cell Therapy for Patients With Metastatic Melanoma: Evaluation of Intensive Myeloablative Chemoradiation Preparative Regimens
Mark E. Dudley, James C. Yang, Richard Sherry, Marybeth S. Hughes, Richard Royal, Udai Kammula,
Paul F. Robbins, JianPing Huang, Deborah E. Citrin, Susan F. Leitman, John Wunderlich, Nicholas P. Restifo,
Armen Thomasian, Stephanie G. Downey, Franz O. Smith, Jacob Klapper, Kathleen Morton,
Carolyn Laurencot, Donald E. White, and Steven A. Rosenberg
Purpose
The two approved treatments for patients with metastatic melanoma, interleukin (IL)-2 and dacarbazine, mediate objective response rates of 12% to 15%. We previously reported that adoptive cell therapy (ACT) with autologous antitumor lymphocytes in lymphodepleted hosts mediated objective responses in 51% of 35 patients. Here, we update that study and evaluate the safety and efficacy of two increased-intensity myeloablative lymphodepleting regimens.
Patients and Method
We performed two additional sequential trials of ACT with autologous tumor-infiltrating lymphocytes (TIL) in patients with metastatic melanoma. Increasing intensity of host preparative lymphodepletion consisting of cyclophosphamide and fludarabine with either 2 (25 patients) or 12 Gy (25 patients) of total-body irradiation (TBI) was administered before cell transfer. Objective
response rates by Response Evaluation Criteria in Solid Tumors (RECIST) and survival were evaluated. Immunologic correlates of effective treatment were studied.
Results
Although nonmyeloablative chemotherapy alone showed an objective response rate of 49%,
when 2 or 12 Gy of TBI was added, the response rates were 52% and 72% respectively.
Responses were seen in all visceral sites including brain. There was one treatment-related death in the 93 patients. Host lymphodepletion was associated with increased serum levels of the lymphocyte homeostatic cytokines IL-7 and IL-15. Objective responses were correlated with the telomere length of the transferred cells.
Conclusion
Host lymphodepletion followed by autologous TIL transfer and IL-2 results in objective responserates of 50% to 70% in patients with metastatic melanoma refractory to standard therapies.
J Clin Oncol 26:5233-5239. Published by the American Society of Clinical Oncology
The paper is upload in my shared files : (see Shared Files)
Dudley JCO '08 Adoptive Cell Therapy for Patients With Metastatic
We have powerful new immunotherapy treatments for patients with metastatic melanoma using cell transfer techniques (see attached publication).
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Steven A. Rosenberg M.D., Ph.D.
Chief, Surgery Branch
National Cancer Institute
10 Center Drive MSC 1201
CRC Room 3-3940
Bethesda, MD 20892
301-496-4164
sar@nih.gov
Adoptive Cell Therapy for Patients With Metastatic Melanoma: Evaluation of Intensive Myeloablative Chemoradiation Preparative Regimens
Mark E. Dudley, James C. Yang, Richard Sherry, Marybeth S. Hughes, Richard Royal, Udai Kammula,
Paul F. Robbins, JianPing Huang, Deborah E. Citrin, Susan F. Leitman, John Wunderlich, Nicholas P. Restifo,
Armen Thomasian, Stephanie G. Downey, Franz O. Smith, Jacob Klapper, Kathleen Morton,
Carolyn Laurencot, Donald E. White, and Steven A. Rosenberg
Purpose
The two approved treatments for patients with metastatic melanoma, interleukin (IL)-2 and dacarbazine, mediate objective response rates of 12% to 15%. We previously reported that adoptive cell therapy (ACT) with autologous antitumor lymphocytes in lymphodepleted hosts mediated objective responses in 51% of 35 patients. Here, we update that study and evaluate the safety and efficacy of two increased-intensity myeloablative lymphodepleting regimens.
Patients and Method
We performed two additional sequential trials of ACT with autologous tumor-infiltrating lymphocytes (TIL) in patients with metastatic melanoma. Increasing intensity of host preparative lymphodepletion consisting of cyclophosphamide and fludarabine with either 2 (25 patients) or 12 Gy (25 patients) of total-body irradiation (TBI) was administered before cell transfer. Objective
response rates by Response Evaluation Criteria in Solid Tumors (RECIST) and survival were evaluated. Immunologic correlates of effective treatment were studied.
Results
Although nonmyeloablative chemotherapy alone showed an objective response rate of 49%,
when 2 or 12 Gy of TBI was added, the response rates were 52% and 72% respectively.
Responses were seen in all visceral sites including brain. There was one treatment-related death in the 93 patients. Host lymphodepletion was associated with increased serum levels of the lymphocyte homeostatic cytokines IL-7 and IL-15. Objective responses were correlated with the telomere length of the transferred cells.
Conclusion
Host lymphodepletion followed by autologous TIL transfer and IL-2 results in objective responserates of 50% to 70% in patients with metastatic melanoma refractory to standard therapies.
J Clin Oncol 26:5233-5239. Published by the American Society of Clinical Oncology
The paper is upload in my shared files : (see Shared Files)
Dudley JCO '08 Adoptive Cell Therapy for Patients With Metastatic
Some Thing New I have found Out about Melanoma!!!! .. Jim Breitfeller
Excerpt from:
"Melanoma invasion – current knowledge and future
directions"
Cedric Gaggioli and Erik Sahai*
Tumour Cell Biology Laboratory, Cancer Research UK, London
Research Institute, London, UK
*Address correspondence to E. Sahai,
e-mail: erik.sahai@cancer.org.uk
"This has dramatic implications
for any attempts to translate any in vitro findings
regarding melanoma cell line invasion into the clinical
situation. The mode of invasion affects the sensitivity of
different cell lines to blockade of MMP function; generally
cells invading with a rounded morphology characteristic
of strong cortical acto-myosin contractility are not
sensitive to MMP inhibition because they can use force
mediated matrix remodelling in place of proteolysis
(Wyckoff et al., 2006)."
"However, cells that do not generate
such high levels of force may be more dependent on
MMP function. When this was directly tested using
WM266.4 cells it was observed that the cells actually
converted to a more rounded MMP independent mode
of invasion (Sahai and Marshall, 2003). Friedl and colleagues
have also demonstrated changes in the mode of
tumour cell invasion in response to blockade of either
protease or integrin beta 1 function (Hegerfeldt et al.,
2002; Wolf et al., 2003)."
"These studies strongly suggest that some melanoma cells can switch their mode of invasion in response to different environmental challenges.
The molecular basis of this plasticity is not well
understood, microarray analyses have demonstrated that
metastatic melanoma cells express genes associated
with a diverse range of cell lineages and this may in part
explain the diverse modes of motility that melanoma
cells can exhibit in vitro (Hendrix et al., 2001)."
" Analysis of clinical samples suggest that melanoma can invade
both collectively and as single cells; however, our understanding
of switching between modes of invasion and
the cytoskeletal regulators critical for the different
modes of invasion is still very limited."
Some Melanoma Cells can Adapt to their Environment. This has major implications based on Dr. Sahai findings.
This may be why Melanoma is so hard to treat.
Jimmy B
"Melanoma invasion – current knowledge and future
directions"
Cedric Gaggioli and Erik Sahai*
Tumour Cell Biology Laboratory, Cancer Research UK, London
Research Institute, London, UK
*Address correspondence to E. Sahai,
e-mail: erik.sahai@cancer.org.uk
"This has dramatic implications
for any attempts to translate any in vitro findings
regarding melanoma cell line invasion into the clinical
situation. The mode of invasion affects the sensitivity of
different cell lines to blockade of MMP function; generally
cells invading with a rounded morphology characteristic
of strong cortical acto-myosin contractility are not
sensitive to MMP inhibition because they can use force
mediated matrix remodelling in place of proteolysis
(Wyckoff et al., 2006)."
"However, cells that do not generate
such high levels of force may be more dependent on
MMP function. When this was directly tested using
WM266.4 cells it was observed that the cells actually
converted to a more rounded MMP independent mode
of invasion (Sahai and Marshall, 2003). Friedl and colleagues
have also demonstrated changes in the mode of
tumour cell invasion in response to blockade of either
protease or integrin beta 1 function (Hegerfeldt et al.,
2002; Wolf et al., 2003)."
"These studies strongly suggest that some melanoma cells can switch their mode of invasion in response to different environmental challenges.
The molecular basis of this plasticity is not well
understood, microarray analyses have demonstrated that
metastatic melanoma cells express genes associated
with a diverse range of cell lineages and this may in part
explain the diverse modes of motility that melanoma
cells can exhibit in vitro (Hendrix et al., 2001)."
" Analysis of clinical samples suggest that melanoma can invade
both collectively and as single cells; however, our understanding
of switching between modes of invasion and
the cytoskeletal regulators critical for the different
modes of invasion is still very limited."
Some Melanoma Cells can Adapt to their Environment. This has major implications based on Dr. Sahai findings.
This may be why Melanoma is so hard to treat.
Jimmy B
Monday, January 19, 2009
Important Advance In The Treatment Of Cancer Melanoma ..Jim Breitfeller!!!
Main Category: Melanoma / Skin Cancer
Also Included In: Lymphoma / Leukemia; Infectious Diseases / Bacteria / Viruses; Genetics
Article Date: 19 Jan 2009 - 2:00 PST
Dr. André Veillette, a researcher at the Institut de recherches cliniques de Montréal (IRCM), and his team led by postdoctoral fellow Dr. Mario-Ernesto Cruz-Munoz, will publish in the upcoming issue of the prestigious journal Nature Immunology of Nature Publishing Group. This discovery could have a significant impact on the treatment of cancers and infectious diseases. Current treatments frequently achieve only limited results with these types of diseases, which affect hundreds of thousands of Canadians.
Dr. Veillette's team identified one of the basic mechanisms controlling NK ("natural killer") cell activity. Produced by the immune system, NK cells are responsible for recognizing and killing cancer cells and cells infected by viruses, such as viruses causing hepatitis and herpes. NK cell deficiency is associated with a higher incidence of cancers and serious infections. "Our breakthrough, comments Dr. Veillette, demonstrates that a molecule known as CRACC, which is present at the surface of NK cells, increases their killer function." Using mice, the researchers have shown that CRACC greatly improves the animals' ability to eliminate cancer cells such as melanoma (a skin cancer) and lymphoma (a blood cancer). Mice lacking the CRACC gene, generated in Dr. Veillette's laboratory, were found to be more susceptible to cancer persistence. Conversely, stimulation of CRACC function was found to improve cancer cell elimination. Thus, stimulating CRACC could boost NK cell activity, helping to fight cancers. In addition, it could improve the ability to fight infections, which are also handled by NK cells.
Increasing the activity of CRACC by gene therapy or drugs could become an option in the future to stimulate the killer function of NK cells, and to improve their capacity to destroy cancer and virus-infected cells. These approaches could be used in combination with chemotherapy and radiotherapy to increase the effectiveness of anti-cancer treatments. Teams of scientists around the world have been trying for many years without success to develop methods to increase NK cell activity. In this light, the discovery of Dr. Veillette's team opens new avenues for the treatment of cancers and viral infections.
This publication constitutes another significant milestone for Dr. Veillette, an internationally renowned immunologist. The article, which is slated for online publication on January 18 in Nature Immunology, gives undeniable evidence for the stimulatory effect of CRACC in NK cells. It is the product of over four years of intensive research by Dr. Veillette's team.
----------------------------
Article adapted by Medical News Today from original press release.
----------------------------
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Canadian Cancer Society and the Howard Hughes Medical Institute (HHMI)
Dr. André Veillette is Director of the Molecular Oncology Research Unit at the IRCM. He holds the Canada Research Chair in Signaling in the Immune System. He is an International Scholar, Howard Hughes Medical Institute (HHMI) and a fellow of the RSC: The Academies of Arts, Humanities and Sciences of Canada. Dr. Veillette is also Professor at the Université de Montréal and Adjunct Professor in the Faculty of Medicine at McGill University.
http://www.ircm.qc.ca
Also Included In: Lymphoma / Leukemia; Infectious Diseases / Bacteria / Viruses; Genetics
Article Date: 19 Jan 2009 - 2:00 PST
Dr. André Veillette, a researcher at the Institut de recherches cliniques de Montréal (IRCM), and his team led by postdoctoral fellow Dr. Mario-Ernesto Cruz-Munoz, will publish in the upcoming issue of the prestigious journal Nature Immunology of Nature Publishing Group. This discovery could have a significant impact on the treatment of cancers and infectious diseases. Current treatments frequently achieve only limited results with these types of diseases, which affect hundreds of thousands of Canadians.
Dr. Veillette's team identified one of the basic mechanisms controlling NK ("natural killer") cell activity. Produced by the immune system, NK cells are responsible for recognizing and killing cancer cells and cells infected by viruses, such as viruses causing hepatitis and herpes. NK cell deficiency is associated with a higher incidence of cancers and serious infections. "Our breakthrough, comments Dr. Veillette, demonstrates that a molecule known as CRACC, which is present at the surface of NK cells, increases their killer function." Using mice, the researchers have shown that CRACC greatly improves the animals' ability to eliminate cancer cells such as melanoma (a skin cancer) and lymphoma (a blood cancer). Mice lacking the CRACC gene, generated in Dr. Veillette's laboratory, were found to be more susceptible to cancer persistence. Conversely, stimulation of CRACC function was found to improve cancer cell elimination. Thus, stimulating CRACC could boost NK cell activity, helping to fight cancers. In addition, it could improve the ability to fight infections, which are also handled by NK cells.
Increasing the activity of CRACC by gene therapy or drugs could become an option in the future to stimulate the killer function of NK cells, and to improve their capacity to destroy cancer and virus-infected cells. These approaches could be used in combination with chemotherapy and radiotherapy to increase the effectiveness of anti-cancer treatments. Teams of scientists around the world have been trying for many years without success to develop methods to increase NK cell activity. In this light, the discovery of Dr. Veillette's team opens new avenues for the treatment of cancers and viral infections.
This publication constitutes another significant milestone for Dr. Veillette, an internationally renowned immunologist. The article, which is slated for online publication on January 18 in Nature Immunology, gives undeniable evidence for the stimulatory effect of CRACC in NK cells. It is the product of over four years of intensive research by Dr. Veillette's team.
----------------------------
Article adapted by Medical News Today from original press release.
----------------------------
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Canadian Cancer Society and the Howard Hughes Medical Institute (HHMI)
Dr. André Veillette is Director of the Molecular Oncology Research Unit at the IRCM. He holds the Canada Research Chair in Signaling in the Immune System. He is an International Scholar, Howard Hughes Medical Institute (HHMI) and a fellow of the RSC: The Academies of Arts, Humanities and Sciences of Canada. Dr. Veillette is also Professor at the Université de Montréal and Adjunct Professor in the Faculty of Medicine at McGill University.
http://www.ircm.qc.ca
Labels:
CRACC gene,
Dr Veillette,
Melanoma,
NK cells,
Université de Montréal
Saturday, January 17, 2009
My search has taken me Overseas!!!! Melanoma .. Jim Breitfeller
My search has taken me Overseas!!!!
Need a copy of "Melanoma invasion - current knowledge and future
directions"
Melanoma invasion - current knowledge and future
directions-- abstract
Erik Sahai PhD
Tumour Cell Biology Laboratory
Cancer Research UK London Research Institute
44 Lincoln's Inn Fields
London
WC2A 3PX
+44 (0)20 72693165
erik.sahai@cancer.org.uk
Dr. was so kind enough to send a copy of paper.
So as I am typing this, I am downloading the paper to my library.
Have a great weekend!!!!
Jimmy B.
Need a copy of "Melanoma invasion - current knowledge and future
directions"
Melanoma invasion - current knowledge and future
directions-- abstract
Erik Sahai PhD
Tumour Cell Biology Laboratory
Cancer Research UK London Research Institute
44 Lincoln's Inn Fields
London
WC2A 3PX
+44 (0)20 72693165
erik.sahai@cancer.org.uk
Dr. was so kind enough to send a copy of paper.
So as I am typing this, I am downloading the paper to my library.
Have a great weekend!!!!
Jimmy B.
Friday, January 16, 2009
Bookmark This Website!! One of the Best for Melanoma Resources.. Jim Breitfeller
Bookmark This Website. This is one of the best sites I have seen for a Melanoma Resource Center:
Mike's Page - The Melanoma Resource Center
Provides translation of links and resources in five languages.
Mike's Page - The Melanoma Resource Center
Have a good weekend!!!!!!!
Mike's Page - The Melanoma Resource Center
Provides translation of links and resources in five languages.
Mike's Page - The Melanoma Resource Center
Have a good weekend!!!!!!!
Educational Teleconference - Cancer Care: New Advances in Melanoma Jim Breitfeller
Date: 1/21/2009
Contact: education@melanoma.org
This teleconference will focus on the new advances in treatment for melanoma. Whether you are newly diagnosed or are years after diagnosis, this program will keep you up-to-date on the new treatments available to melanoma patients. The conference is intended for patients with melanoma along with their friends, or family members.
Speaker: Dr. Keith Flaherty, Assistant Professor of Medicine, Abramson Cancer Center of the University of Pennsylvania
Moderator: Dr. Lynn Schuchter, Professor of Medicine, Abramson Cancer Center of the University of Pennsylvania
Join us for a free teleconference, Cancer Care: New Advances in Treatment, from 12:00 pm to 1:00 pm Eastern Daylight Time (EDT) on January 21, 2009. After Dr. Flaherty’s presentation, there will be time for live question and answer period.
Melanoma Educational Teleconference
Click on link and register today.
I have read some of Dr. Flaherty's papers
Description of Research Expertise
Experimental therapies
Description of Clinical Expertise
Melanoma
Renal cell carcinoma
Selected Publications
Flaherty KT, Malkowicz SB, Vaughn DJ: A Phase I Study of Weekly Liposome-encapsulated Doxorubicin in Patients with Advanced, Androgen-independent Prostate Cancer. Am J Clin Oncol. 27(2): 136-9, Apr 2004.
Flaherty KT, Stevenson JP, Redlinger M, Giantonio B, Algazy KM, O’Dwyer PJ: A Phase I, Dose Escalation Trial of ZD0473, a Novel Platinum Analogue, in Combination with Gemcitabine. Cancer Chemother Pharmacol. Jan 29 2004.
Bilenker JH, Stevenson JP, Flaherty KT, Algazy K, McLaughlin K, Haller DG, Giantonio BJ, Koehler M, Garcia-Vargas JE, O'Dwyer PJ. : Phase I trial of the antifolate ZD9331 in combination with cisplatin in patients with refractory solid malignancies. Cancer Chemother Pharmacol. 53(4): 357-60, Apr 2004.
Flaherty KT: New molecular targets in melanoma. Curr Opin Oncol. 16(2): 150-4, Mar 2004.
Flaherty KT., Stevenson JP., Hahn SM., Redlinger M., O'Dwyer PJ.: Phase I combination trial of gemcitabine, paclitaxel, and carboplatin in patients with advanced malignancy. Cancer Chemotherapy & Pharmacology 52(3): 217-22, Sep 2003.
Flaherty KT., Stevenson JP., Gallagher M., Giantonio B., Algazy KM., Sun W., Haller DG., O'Dwyer PJ.: Dose escalation study of tezacitabine in combination with cisplatin in patients with advanced cancer. Cancer 97(8): 1985-90, Apr 15 2003.
Flaherty KT, Tuveson D, Brose M, Rosen M, Lee RJ, Schwartz B, Schuchter LM, O’Dwyer PJ: Pharmacodynamic Study of BAY 43-9006 in Patients with Renal Cell Carcinoma and Melanoma. NCI-AACR-EORTC 2003, poster #A84. 2003.
Flaherty KT, Lee RJ, Humphrey R, O’Dwyer PJ, Schiller J: Phase I trial of BAY 43-9006 in combination with carboplatin and paclitaxel. ASCO 2003, oral presentation, abstract #2854 2003.
Flaherty KT, O’Dwyer PJ: Phase II trials: Conventional Design and Novel Strategies in the Era of Targeted Therapy. Anticancer Drug Development Guide. Teicher BA (eds.). Feb 2003 Notes: In press.
Tao J., Flaherty K., Bagg A.: Unusual hematologic malignancies. Case 1. Hematologic malignancy presenting with diarrhea and bony lesions: systemic mastocytosis. Journal of Clinical Oncology 20(17): 3737-9, Sep 1 2002.
Last updated: 08/20/2007
The Trustees of the University of Pennsylvania
Jimmy B
Contact: education@melanoma.org
This teleconference will focus on the new advances in treatment for melanoma. Whether you are newly diagnosed or are years after diagnosis, this program will keep you up-to-date on the new treatments available to melanoma patients. The conference is intended for patients with melanoma along with their friends, or family members.
Speaker: Dr. Keith Flaherty, Assistant Professor of Medicine, Abramson Cancer Center of the University of Pennsylvania
Moderator: Dr. Lynn Schuchter, Professor of Medicine, Abramson Cancer Center of the University of Pennsylvania
Join us for a free teleconference, Cancer Care: New Advances in Treatment, from 12:00 pm to 1:00 pm Eastern Daylight Time (EDT) on January 21, 2009. After Dr. Flaherty’s presentation, there will be time for live question and answer period.
Melanoma Educational Teleconference
Click on link and register today.
I have read some of Dr. Flaherty's papers
Description of Research Expertise
Experimental therapies
Description of Clinical Expertise
Melanoma
Renal cell carcinoma
Selected Publications
Flaherty KT, Malkowicz SB, Vaughn DJ: A Phase I Study of Weekly Liposome-encapsulated Doxorubicin in Patients with Advanced, Androgen-independent Prostate Cancer. Am J Clin Oncol. 27(2): 136-9, Apr 2004.
Flaherty KT, Stevenson JP, Redlinger M, Giantonio B, Algazy KM, O’Dwyer PJ: A Phase I, Dose Escalation Trial of ZD0473, a Novel Platinum Analogue, in Combination with Gemcitabine. Cancer Chemother Pharmacol. Jan 29 2004.
Bilenker JH, Stevenson JP, Flaherty KT, Algazy K, McLaughlin K, Haller DG, Giantonio BJ, Koehler M, Garcia-Vargas JE, O'Dwyer PJ. : Phase I trial of the antifolate ZD9331 in combination with cisplatin in patients with refractory solid malignancies. Cancer Chemother Pharmacol. 53(4): 357-60, Apr 2004.
Flaherty KT: New molecular targets in melanoma. Curr Opin Oncol. 16(2): 150-4, Mar 2004.
Flaherty KT., Stevenson JP., Hahn SM., Redlinger M., O'Dwyer PJ.: Phase I combination trial of gemcitabine, paclitaxel, and carboplatin in patients with advanced malignancy. Cancer Chemotherapy & Pharmacology 52(3): 217-22, Sep 2003.
Flaherty KT., Stevenson JP., Gallagher M., Giantonio B., Algazy KM., Sun W., Haller DG., O'Dwyer PJ.: Dose escalation study of tezacitabine in combination with cisplatin in patients with advanced cancer. Cancer 97(8): 1985-90, Apr 15 2003.
Flaherty KT, Tuveson D, Brose M, Rosen M, Lee RJ, Schwartz B, Schuchter LM, O’Dwyer PJ: Pharmacodynamic Study of BAY 43-9006 in Patients with Renal Cell Carcinoma and Melanoma. NCI-AACR-EORTC 2003, poster #A84. 2003.
Flaherty KT, Lee RJ, Humphrey R, O’Dwyer PJ, Schiller J: Phase I trial of BAY 43-9006 in combination with carboplatin and paclitaxel. ASCO 2003, oral presentation, abstract #2854 2003.
Flaherty KT, O’Dwyer PJ: Phase II trials: Conventional Design and Novel Strategies in the Era of Targeted Therapy. Anticancer Drug Development Guide. Teicher BA (eds.). Feb 2003 Notes: In press.
Tao J., Flaherty K., Bagg A.: Unusual hematologic malignancies. Case 1. Hematologic malignancy presenting with diarrhea and bony lesions: systemic mastocytosis. Journal of Clinical Oncology 20(17): 3737-9, Sep 1 2002.
Last updated: 08/20/2007
The Trustees of the University of Pennsylvania
Jimmy B
I got the "Clean and Green" from Dr. Marino .. Jim Breitfeller Melanoma
Well things look good so far. I do need to have my blood tested but I have to fast first so I will do that next week. So, I am back here with you trying to work out the puzzle.
Have a Great Weekend, My happy hour has started!!!!
Dinner = fish with Spinach
Desert = 2 bx of sugar free pudding Vanilla
1 container cool whip lite
1 can crushed pineapple 12 to 16 oz
1 pie crust (baked)
Mix together and refrigerate
ENJOY
jIMMY b
Have a Great Weekend, My happy hour has started!!!!
Dinner = fish with Spinach
Desert = 2 bx of sugar free pudding Vanilla
1 container cool whip lite
1 can crushed pineapple 12 to 16 oz
1 pie crust (baked)
Mix together and refrigerate
ENJOY
jIMMY b
I am coming up for Air!!!
I am coming up for Air!!!
I have a Dr. appointment with my PCP (Dr. Marino)tomorrow at 10:30.
It is a check to see if eveything is still going well. I can't complain, see I've been jucing vegie's. I need all the protiens and Vitamins that I can. It gives me a sense that I am doing all I can to extend my journey here on earth.
Take care
Jimmy B
I have a Dr. appointment with my PCP (Dr. Marino)tomorrow at 10:30.
It is a check to see if eveything is still going well. I can't complain, see I've been jucing vegie's. I need all the protiens and Vitamins that I can. It gives me a sense that I am doing all I can to extend my journey here on earth.
Take care
Jimmy B
I Thought I was the Only ONE Seeing This Trend!! Melanoma Jim Breitfeller
Sorry, I tried to catch up on some reading and this was in the pile.
I thought it could not wait. I am Vindicated!!!!
Promising immunotherapeutic approaches to the treatment of metastatic melanoma: modulation of the immune response
Mario Sznol, MD
Yale Cancer Center, New Haven, CT
Durable remissions of advanced unresectable melanoma can be achieved in a small fraction of patients with immune-based therapy, such as high-dose interleukin-2 (IL-2). Based on an increased understanding of normal and pathologic immune responses at the molecular level and the specific interactions between growing tumors and the host immune response, new approaches to cancer immunotherapy are emerging. Optimism for advances in the immunotherapy of melanoma comes from new agents and approaches that specifically target and modify the regulators of T-cell proliferation, survival and effector function, and/or block tumor-related immunosuppressive mechanisms. One of these agents, anti-CTLA4 (anti-cytotoxic T-lymphocyte–associated antigen 4), has shown the ability to produce durable responses in patients with metastatic melanoma, including some previously unresponsive to IL-2. Preclinical studies indicate that even stronger antitumor immune responses can evolve when agents are combined rationally.
Promising immunotherapeutic approaches to the treatment of metastatic melanoma: modulation of the immune response
I shouted FIRE and no one came. Well, now there is two of us Shouting!!!!!
Jimmy B
I thought it could not wait. I am Vindicated!!!!
Promising immunotherapeutic approaches to the treatment of metastatic melanoma: modulation of the immune response
Mario Sznol, MD
Yale Cancer Center, New Haven, CT
Durable remissions of advanced unresectable melanoma can be achieved in a small fraction of patients with immune-based therapy, such as high-dose interleukin-2 (IL-2). Based on an increased understanding of normal and pathologic immune responses at the molecular level and the specific interactions between growing tumors and the host immune response, new approaches to cancer immunotherapy are emerging. Optimism for advances in the immunotherapy of melanoma comes from new agents and approaches that specifically target and modify the regulators of T-cell proliferation, survival and effector function, and/or block tumor-related immunosuppressive mechanisms. One of these agents, anti-CTLA4 (anti-cytotoxic T-lymphocyte–associated antigen 4), has shown the ability to produce durable responses in patients with metastatic melanoma, including some previously unresponsive to IL-2. Preclinical studies indicate that even stronger antitumor immune responses can evolve when agents are combined rationally.
Promising immunotherapeutic approaches to the treatment of metastatic melanoma: modulation of the immune response
I shouted FIRE and no one came. Well, now there is two of us Shouting!!!!!
Jimmy B
Can you Say Pharmacogenomics & Personalized Medicine!!!! Melanoma Jim Breitfeller
Pharmacogenomics & Personalized Medicine
Dr.Markovic gave us Help with the PUZZLE. We need to take into account these three elements as he called them.
"basically that there are 3 elements to the puzzle that need to be addressed: (1) the tumor; (2) its vascular supply; and (3) the body's immune system."
It go me thinking,Thats right, I was up half the night trying find the right angle to approach the puzzle.
The commonality was the patient!!!!! What do we have that the Oncologist needs to know to send us on the right Therapy?
He needs to know which patient will clinically respond to the therapy that they have chosen.
Sooooo!!!! Then it came to Me "Molecular Diagnositics that lead me to Pharmacogenomics.
You might not hear from me for awhile, until I come up for Air.
I need to do some research/HOMEWORK!!!!!!!
Prognostic and Predictive Molecular Markers in Cutaneous Malignant Melanoma
Jimmy B
Dr.Markovic gave us Help with the PUZZLE. We need to take into account these three elements as he called them.
"basically that there are 3 elements to the puzzle that need to be addressed: (1) the tumor; (2) its vascular supply; and (3) the body's immune system."
It go me thinking,Thats right, I was up half the night trying find the right angle to approach the puzzle.
The commonality was the patient!!!!! What do we have that the Oncologist needs to know to send us on the right Therapy?
He needs to know which patient will clinically respond to the therapy that they have chosen.
Sooooo!!!! Then it came to Me "Molecular Diagnositics that lead me to Pharmacogenomics.
You might not hear from me for awhile, until I come up for Air.
I need to do some research/HOMEWORK!!!!!!!
Prognostic and Predictive Molecular Markers in Cutaneous Malignant Melanoma
Jimmy B
Metastatic melanoma—challenges for the community oncologist!! Jim Breitfeller
Michael B. Atkins, MD | Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA
"The outlook for patients with advanced
melanoma is bleak. Patients with stage IV melanoma have a median
survival of 6–9 months,1–3 with 5-year survival rates of 1%–2%.1,4
An estimated 8,000 Americans will die of melanoma in 2008.1,5 Many of these patients are young, with a median age of ~ 50 years, and otherwise healthy.1,5 This loss of life represents a societal burden in excess
of the actual numbers. Although melanoma is the ninth most common cancer in the United States,1,6 it ranks second among solid tumors in terms of years of productive life lost.
Common treatment approaches have included:
cytotoxic chemotherapy; interleukin-2 (IL-2)–based immunotherapy; and combinations of chemotherapy and immunotherapy (so-called biochemotherapy). Although some treatment approaches, most notably, high-dose IL-2 immunotherapy,
have been shown to produce extremely durable tumor responses in a small percentage of patients,4,7,8 no treatment approach has shown a reproducible survival advantage in phase III trials.
There is a critical need for new treatment approaches for patients with advanced melanoma.Recent advances in the understanding of melanoma
biology, immune regulation, and tumor-induced immune suppression are the foundation for new opportunities to improve therapeutic outcomes for patients with advanced melanoma. Studies of melanoma biology have uncovered critical factors that are important for melanoma cell survival, including activating mutations in BRAF, which occur in > 50% of melanomas; mutations in KIT, the gene encoding the c-Kit receptor, which are common in acral lentiginous and mucosal primary melanomas; overexpression of vascular endothelial growth factor, which drives tumor angiogenesis; and an assortment of factors that limit the apoptotic effects of various cytotoxic chemotherapy agents."
Read the rest:
http://www.oncologystat.com/Images/metastatic_melanoma_tcm8-162554.pdf
Metastatic melanoma—challenges for the community oncologist
Jimmy B
"The outlook for patients with advanced
melanoma is bleak. Patients with stage IV melanoma have a median
survival of 6–9 months,1–3 with 5-year survival rates of 1%–2%.1,4
An estimated 8,000 Americans will die of melanoma in 2008.1,5 Many of these patients are young, with a median age of ~ 50 years, and otherwise healthy.1,5 This loss of life represents a societal burden in excess
of the actual numbers. Although melanoma is the ninth most common cancer in the United States,1,6 it ranks second among solid tumors in terms of years of productive life lost.
Common treatment approaches have included:
cytotoxic chemotherapy; interleukin-2 (IL-2)–based immunotherapy; and combinations of chemotherapy and immunotherapy (so-called biochemotherapy). Although some treatment approaches, most notably, high-dose IL-2 immunotherapy,
have been shown to produce extremely durable tumor responses in a small percentage of patients,4,7,8 no treatment approach has shown a reproducible survival advantage in phase III trials.
There is a critical need for new treatment approaches for patients with advanced melanoma.Recent advances in the understanding of melanoma
biology, immune regulation, and tumor-induced immune suppression are the foundation for new opportunities to improve therapeutic outcomes for patients with advanced melanoma. Studies of melanoma biology have uncovered critical factors that are important for melanoma cell survival, including activating mutations in BRAF, which occur in > 50% of melanomas; mutations in KIT, the gene encoding the c-Kit receptor, which are common in acral lentiginous and mucosal primary melanomas; overexpression of vascular endothelial growth factor, which drives tumor angiogenesis; and an assortment of factors that limit the apoptotic effects of various cytotoxic chemotherapy agents."
Read the rest:
http://www.oncologystat.com/Images/metastatic_melanoma_tcm8-162554.pdf
Metastatic melanoma—challenges for the community oncologist
Jimmy B
Labels:
C kit testing,
CTLA-4,
Dr. Atkins,
IL-2,
interlukin-2,
Melanoma,
survival rates,
V600E BRAF mutation
You can Join too!!!!! "OncologySTAT" Melanoma jim Breitfeller
Hello Mr Breitfeller,
Thank you for registering with OncologySTAT. You are now able to access the following high value clinical tools and information:
- Search and download current articles from 100+ Elsevier cancer related journals.
- Literature scans from the top 25 cancer related journals (JCO, JNCI, CA, Blood, NEJM, JAMA, etc.)
- Daily medical and regulatory news from Elsevier Global Medical News Group and FDC Reports' "The Pink Sheet Daily."
- 29 Cancer Type Spotlights (Breast, Lung, Prostate, etc)
- Professional Drug Monograph and Interactions Database; patient handouts
- Chemotherapy Regimens (The Elsevier Guide to Oncology Drugs and Regimens, 2006 edition)
- Coverage of all major cancer conferences & meetings
- Plus MEDLINE, expert interviews, blogs, videos, and more
Thank you,
OncologySTAT
Thank you for registering with OncologySTAT. You are now able to access the following high value clinical tools and information:
- Search and download current articles from 100+ Elsevier cancer related journals.
- Literature scans from the top 25 cancer related journals (JCO, JNCI, CA, Blood, NEJM, JAMA, etc.)
- Daily medical and regulatory news from Elsevier Global Medical News Group and FDC Reports' "The Pink Sheet Daily."
- 29 Cancer Type Spotlights (Breast, Lung, Prostate, etc)
- Professional Drug Monograph and Interactions Database; patient handouts
- Chemotherapy Regimens (The Elsevier Guide to Oncology Drugs and Regimens, 2006 edition)
- Coverage of all major cancer conferences & meetings
- Plus MEDLINE, expert interviews, blogs, videos, and more
Thank you,
OncologySTAT
Wednesday, January 14, 2009
A Reply back from DR. Markovic, Svetomir N., M.D., Ph.D. ON Emerging Therapies for Melanoma ..Jim Breitfeller
Emerging Therapies for Melanoma
Rajini Katipamula; Svetomir N Markovic
Expert Rev Anticancer Ther. 2008;8(4):553-560.
Posted 07/09/2008
Dear Mr. Breitfeller,
Thank you for your note.
I just got back from a trip and am catching up with e-mail. I must say, I really enjoyed reading your message.
Our thoughts on the matter (as summarized in my brief article you noted) are basically that there are 3 elements to the puzzle that need to be addressed:
(1) the tumor; (2) its vascular supply; and (3) the body's immune system.
Addressing each one of these alone, will only gain little benefits for most, transient benefits for some and awesome results in a lucky few patients. So, why not look at the problem in the context of the patient.
And, that is basically where we are right now. We are crafting therapies that will engage all 3 elements; we measure the impact on all 3 and gain insights into what's working, what's not, and what we need to do next.
The first study we did using this approach was just published in Cancer this month. And, we did reasonably well for the majority of patients (best progression free survival result in a single arm phase II study done by the US cooperative groups in patients with metastatic melanoma).
What was most interesting is that we learned that the drugs we used may have worked in a completely different mechanism... The combination of chemotherapy and an inhibitor of angiogenesis may have yielded the good clinical outcomes via an immunological mechanism (paper in preparation).
I think that there may be something to this, and we'll keep plugging on this path. Hopefully we will be able to lend some insights that may be helpful to our patients and colleagues.
Take care,
Svetomir
--------------------------------------------------------------------------------
From: dbreitfe [mailto:dbreitfe@rochester.rr.com]
Sent: Monday, January 05, 2009 8:28 AM
To: Markovic, Svetomir N., M.D., Ph.D.
Subject: permission to post on Carepages "Emerging Therapies for Melanoma"
Svetomir, I am a cancer patient under the care of John M Kirkwood at the Hillman Cancer Center. I think you paper is very fascinating. Let me give you a little back ground of my dealings with Melanoma:
Last July (2005)I was riding my bicycle to work at the Eastman Kodak Research Labs about 3 miles from home. I was wearing a knapsack to carry my things to and from the labs. I started noticing an ache on my back. I asked my wife to take a look at it. It was just a mole. So I decide to go to the dermatologist. To make the log story short, it was cancer. It took about a month and a half to get it biopsied . (Sept. 26 2005) I contacted a surgical oncologist and on October 27 2005 I had a wide incision to remove the tumor off my back. I also had a PET scan and a sentinel lymph node biopsy done and it showed no cancer except the localized one on my back. I knew from my research that I would be needing adjuvant therapy.
So I started communicating with Sloan Kettering, University of Pittsburgh Cancer Center, and a couple of others including the Wilmot Cancer Center at Strong. They all recommended to start with Interferon treatment. In the meantime, my back got infected and it took until Feb. 14 2006 to heal. On March 6 2006, I started the High Dose Interferon treatment that was to last 4 weeks with daily infusions. The treatment lasted only 2 ½ weeks because my blood sugar went completely out of control. I also have type 2 diabetes.
About 2 weeks after the interferon treatments, I noticed my right lymph node growing to golf ball size and they were aching. I had them biopsied (April 4 2006) and 9 out of 11 nodes were cancerous. On April 24th , I had my lump nodes removed under my right arm. This had move me from a stage 3 to a stage 4. The cancer was spreading quite rapidly. Strong did not have any specialists in the field of malignant melanoma. So, I was in contact with Dr. John Kirkwood in Pittsburgh. So with my oncologist approval, my wife and I went down to Pittsburgh and had a consultation with Dr. Kirkwood (June 12, 2006) Kirkwood wanted another PET scan (June 16 2006) to see if the cancer was contained to my lymph nodes. The results showed 4 tumors on my back, one under my right arm, and two spots, one in each lobe of my lungs. It wasn’t good.
So, On July 10, 2006, I started my clinical trial of Dicarbazine and Patrin cycle 1. Each cycle is 21 days. On day 3, I am injected with dicarbazine at the Hillman Cancer Center in Pittsburgh. On the 11th day I go back to have a check up. Twice a month I traveled to Pittsburgh at least with this trial. I started my 2nd cycle on July 31 2006. On August 17 I had a CT scan and the report showed new tumors in my lungs so they stopped the Dicarbazine trial.
On September 6th, I had another CT scan and MRI for the next trial which was Anti-CTLA-4. On September 13 I had my first infusion. On October 8, 2006 My wife noticed two new growths on my back. It was confirmed on October 11th it was new tumors.
Dr. John Kirkwood has decided that IL 2 Interlukin 2 would be next course of action. I have completed another round of tests (CT scan, Pulmonary Function, and a Nuclear Stress Test). The CT scans shows 40+ nodules in my lungs ranging from 15mm to less than 5mm.
I am slated to be High dosage IL-2 on November 1st 2006.
I am presently washed out an IL-2 clinical trial that started in November 1, 2006. On the fourth cycle I had a heart attack and the doctors determined to abort the IL-2 on February 2, 2007. On August 23 2007 there was no change to the tumors in my back or lungs but also no growth. In October 24 2007 I got the word that the tumors and the lung nodules were shrinking. In April 14, 2008, the 40 + nodules in my lungs decrease to 2. In July 2008, the nodules in my lungs were undetectable and the ones on my back were all but one gone. Presently, CT and MRI
In November 2008 show no signs cancerous activity. I am stabilized for the time being.
As you can see I did the CTLA-4, one dose and then went on to IL-2 until I had a heart attack. Base on your 5 year View, in my case, my overall survival rate has doubled so far.
“Five-year View”
“Several advances are being made in the field of immunological and targeted therapies. In our opinion, the agents of most interest in the next 5 years would be the anti-CTLA-4 antibodies and angiogenesis inhibitors combined with cytotoxic therapy. While most of these novel agents may not have significant single-agent activity, combining them with systemic chemotherapy might be the key to clinical success in melanoma. Ultimately, rational combination therapeutics of multimodal targeting agents (immunotherapeutics, cytotoxic therapy, angiogenesis inhibitors) will likely lead to substantive clinical benefit in this patient population. Our hope is that this massive clinical research endeavor will soon yield the long sought after improvement in the overall survival of patients with advanced melanoma.”
Being a researcher myself, I came up with a theory.
11/06/08 This is my Theory
Posted Nov 6, 2008 9:59am
11/06/08 This is my Theory
The Interlukin-2
IL-2, which works by stimulating killer T-cells to attack melanoma. In some cases with the right body chemistry, helps communicate that message to the killer T-cells and the body begins to fight off the Beast.
In other cases, your body chemistry is different than mine and may lack some sort protein/? or what ever. So in this case, the communication is lost like a drop signal from your cell phone. What you need is another Cell Tower to transmit that signal. That is where CTLA-4 coming in to play. It builds the tower to help with the communication. I may be over simplifying the biochemistry but I am not in the lab to do the right experiments. So, I have to take an educated guess.
So, I did a little online research
As you can see, Dr. Kirkwood and I wanted to induce tumor regression by using my own immune system. If we could get my immune system to recognize the tumors as foreign, then we might have a fighting chance.
So we decide to try the CTLA-4 Therapy, Antitumor response with prolonged time to progression has been seen in patients with melanoma who have received either of the CTLA-4 antibodies and durable antitumor responses have been observed with ipilimumab in patients with melanoma ovarian cancer, prostate cancer, and renal cell carcinoma It has been seen, antitumor responses may be characterized by short-term progression followed by delayed regression.
An important, possibly unique, clinical characteristic of anti-CTLA-4 antibodies is that the duration of clinical response -- and even stable disease -- is often quite prolonged.
This is what I believe is going on in my case but I have no proof.
So lets combine the two clinical trials and that was done by Dr. Rosenberg at the National Cancer Institute. This did not yield the Better outcome.
I think it is because doing the therapy at the same time my be jamming the signals or changing the micro-chemical in the cell and surrounding environment so that it is not inductive to passing on the communication cell to cell.. protein to protein .
But since there was a lag between my two therapies, My cells began to communicate and jump started my immune system. Do I think is a one off, NO!!!!! I have been in touch with another patient that did IL-2 then, CTLA-4 and got the same response. The trick is to get the timing and the dose right!!!!!
So I took it apon myself to to a little research and this is what I came up with. I believe it all makes sense. I still may be over simplifying the actual process but I am not a biochemist. So Here goes:
Dendritic cells (DCs) are immune cells and form part of the our immune system. Their main function is to process antigen material and present it on the surface to other cells of the immune system, thus functioning as Antigen-Presenting Cells (APC).
The dendritic cells are constantly in communication with other cells in the body. This communication can take the form of direct cell-to-cell contact based on the interaction of cell-surface proteins. An example of this includes the interaction of the receptor B7 of the dendritic cell with CD28 present on the lymphocyte. However, the cell-cell interaction can also take place at a distance via cytokines. These components of the immune system communicate with one another by exchanging chemical messengers. These proteins are secreted by cells and act on other cells to coordinate an appropriate immune response.
Cytokines include a diverse assortment of interleukins, interferons, and growth factors.One cytokine, interleukin 2 (IL-2), triggers the immune system to produce T cells. IL-2’s immunity-boosting properties have traditionally made it a promising treatment for several illnesses which include Hepatitis C and Melanoma.
There are several steps to activation of the immune system against a foreign molecule. The T cell receptor must first interact with the MHC molecule. The T cell receptor or TCR is a molecule found on the surface of T lymphocytes (or T cells) that is, in general, responsible for recognizing antigens bound to Major Histocompatibility Complex (MHC) molecules. MHC the most gene-dense region of the Human genome and plays an important role in the immune system, autoimmunity.
This first interaction involves the CD4 or CD8 proteins which form a complex with the CD3 protein to bind to the MHC molecule of the (APC). Antigen-presenting cell This is also called "Signal 1" and its main purpose is T cell activation.
However, this is insufficient for producing a T cell response by itself. In fact, lack of further stimulatory signals sends the T cell into anergy. Anergy is a term in immunobiology that describes a lack of reaction by the body's defense mechanisms to foreign substances.
The Second costimulatory signal necessary to continue the immune response can come from B7-CD28 and CD40-CD40L interactions. The primary role of the B7 proteins is to give a second signal to the T cell. The B7 protein/receptor is present on the Antigen-presenting cell and is able to interact with the CD28 receptor on the T cell surface; this is also known as "Signal 2". There are other activation signals which play a role in immune responses.
On these T cells there is are family receptors whose job is downregulate the T cell activation so the immune system maintains metabolic equilibrium so the immune system doesn’t start an autoimmune response and cause it to attack itself. One of these receptors is Cytotoxic T lymphocyte-associated antigen (CTLA4).
It was hypothesized back in the 1980’s that if you replaced the CTLA4 with Anti-CTLA4 that it might block the B7 receptor causing an enhancement of the T-cell activation, leading to a more robust antitumor immune response.
It was shown in mice with a disrupted CTLA-4 genes that their immune response ran unabated causing autoimmunity which was fatal.
It was also shown that the anti-CTLA-4 antibodies had a greater affinity to CTLA-4 than the B7 receptor. So by doing the CTLA-4 Therapy, it allowed signal 1 to become active.
So in the presents of the CTLA-4 antibody Therapy, I my case, we may have extended the antitumor response of the T-cells. This left Signal 1 active.
We then, hit the immune system with High dose of IL-2. This must have stimulated the cell to cell communication (Signal 2) causing the immune response to kick in against the foreign molecule (The Tumor)
AS YOU GUESSED IT, I MUST HAVE JUMP STARTED MY IMMUNE SYSTEM!!!!!!
Just don't know how long it will last.
AS you can see , I am passionate about finding a cure. My Life depends on it. So if I can pass along information that may help others, I do. And that is why I am asking for your Permission on post this information.
I hope to here from you soon
Thanks for your time and Keep the good research coming!!!!
Sincerely,
James M. Breitfeller
The information contained is intended for the confidential use of the intended recipient(s) only and may be legally privileged. If it has been received other than by an intended recipient, it may not be reviewed, disseminated, distributed, copied or retained. Therefore, please notify the sender immediately and delete the original message and all copies.
Thank you
James M. Breitfeller
Rajini Katipamula; Svetomir N Markovic
Expert Rev Anticancer Ther. 2008;8(4):553-560.
Posted 07/09/2008
Dear Mr. Breitfeller,
Thank you for your note.
I just got back from a trip and am catching up with e-mail. I must say, I really enjoyed reading your message.
Our thoughts on the matter (as summarized in my brief article you noted) are basically that there are 3 elements to the puzzle that need to be addressed:
(1) the tumor; (2) its vascular supply; and (3) the body's immune system.
Addressing each one of these alone, will only gain little benefits for most, transient benefits for some and awesome results in a lucky few patients. So, why not look at the problem in the context of the patient.
And, that is basically where we are right now. We are crafting therapies that will engage all 3 elements; we measure the impact on all 3 and gain insights into what's working, what's not, and what we need to do next.
The first study we did using this approach was just published in Cancer this month. And, we did reasonably well for the majority of patients (best progression free survival result in a single arm phase II study done by the US cooperative groups in patients with metastatic melanoma).
What was most interesting is that we learned that the drugs we used may have worked in a completely different mechanism... The combination of chemotherapy and an inhibitor of angiogenesis may have yielded the good clinical outcomes via an immunological mechanism (paper in preparation).
I think that there may be something to this, and we'll keep plugging on this path. Hopefully we will be able to lend some insights that may be helpful to our patients and colleagues.
Take care,
Svetomir
--------------------------------------------------------------------------------
From: dbreitfe [mailto:dbreitfe@rochester.rr.com]
Sent: Monday, January 05, 2009 8:28 AM
To: Markovic, Svetomir N., M.D., Ph.D.
Subject: permission to post on Carepages "Emerging Therapies for Melanoma"
Svetomir, I am a cancer patient under the care of John M Kirkwood at the Hillman Cancer Center. I think you paper is very fascinating. Let me give you a little back ground of my dealings with Melanoma:
Last July (2005)I was riding my bicycle to work at the Eastman Kodak Research Labs about 3 miles from home. I was wearing a knapsack to carry my things to and from the labs. I started noticing an ache on my back. I asked my wife to take a look at it. It was just a mole. So I decide to go to the dermatologist. To make the log story short, it was cancer. It took about a month and a half to get it biopsied . (Sept. 26 2005) I contacted a surgical oncologist and on October 27 2005 I had a wide incision to remove the tumor off my back. I also had a PET scan and a sentinel lymph node biopsy done and it showed no cancer except the localized one on my back. I knew from my research that I would be needing adjuvant therapy.
So I started communicating with Sloan Kettering, University of Pittsburgh Cancer Center, and a couple of others including the Wilmot Cancer Center at Strong. They all recommended to start with Interferon treatment. In the meantime, my back got infected and it took until Feb. 14 2006 to heal. On March 6 2006, I started the High Dose Interferon treatment that was to last 4 weeks with daily infusions. The treatment lasted only 2 ½ weeks because my blood sugar went completely out of control. I also have type 2 diabetes.
About 2 weeks after the interferon treatments, I noticed my right lymph node growing to golf ball size and they were aching. I had them biopsied (April 4 2006) and 9 out of 11 nodes were cancerous. On April 24th , I had my lump nodes removed under my right arm. This had move me from a stage 3 to a stage 4. The cancer was spreading quite rapidly. Strong did not have any specialists in the field of malignant melanoma. So, I was in contact with Dr. John Kirkwood in Pittsburgh. So with my oncologist approval, my wife and I went down to Pittsburgh and had a consultation with Dr. Kirkwood (June 12, 2006) Kirkwood wanted another PET scan (June 16 2006) to see if the cancer was contained to my lymph nodes. The results showed 4 tumors on my back, one under my right arm, and two spots, one in each lobe of my lungs. It wasn’t good.
So, On July 10, 2006, I started my clinical trial of Dicarbazine and Patrin cycle 1. Each cycle is 21 days. On day 3, I am injected with dicarbazine at the Hillman Cancer Center in Pittsburgh. On the 11th day I go back to have a check up. Twice a month I traveled to Pittsburgh at least with this trial. I started my 2nd cycle on July 31 2006. On August 17 I had a CT scan and the report showed new tumors in my lungs so they stopped the Dicarbazine trial.
On September 6th, I had another CT scan and MRI for the next trial which was Anti-CTLA-4. On September 13 I had my first infusion. On October 8, 2006 My wife noticed two new growths on my back. It was confirmed on October 11th it was new tumors.
Dr. John Kirkwood has decided that IL 2 Interlukin 2 would be next course of action. I have completed another round of tests (CT scan, Pulmonary Function, and a Nuclear Stress Test). The CT scans shows 40+ nodules in my lungs ranging from 15mm to less than 5mm.
I am slated to be High dosage IL-2 on November 1st 2006.
I am presently washed out an IL-2 clinical trial that started in November 1, 2006. On the fourth cycle I had a heart attack and the doctors determined to abort the IL-2 on February 2, 2007. On August 23 2007 there was no change to the tumors in my back or lungs but also no growth. In October 24 2007 I got the word that the tumors and the lung nodules were shrinking. In April 14, 2008, the 40 + nodules in my lungs decrease to 2. In July 2008, the nodules in my lungs were undetectable and the ones on my back were all but one gone. Presently, CT and MRI
In November 2008 show no signs cancerous activity. I am stabilized for the time being.
As you can see I did the CTLA-4, one dose and then went on to IL-2 until I had a heart attack. Base on your 5 year View, in my case, my overall survival rate has doubled so far.
“Five-year View”
“Several advances are being made in the field of immunological and targeted therapies. In our opinion, the agents of most interest in the next 5 years would be the anti-CTLA-4 antibodies and angiogenesis inhibitors combined with cytotoxic therapy. While most of these novel agents may not have significant single-agent activity, combining them with systemic chemotherapy might be the key to clinical success in melanoma. Ultimately, rational combination therapeutics of multimodal targeting agents (immunotherapeutics, cytotoxic therapy, angiogenesis inhibitors) will likely lead to substantive clinical benefit in this patient population. Our hope is that this massive clinical research endeavor will soon yield the long sought after improvement in the overall survival of patients with advanced melanoma.”
Being a researcher myself, I came up with a theory.
11/06/08 This is my Theory
Posted Nov 6, 2008 9:59am
11/06/08 This is my Theory
The Interlukin-2
IL-2, which works by stimulating killer T-cells to attack melanoma. In some cases with the right body chemistry, helps communicate that message to the killer T-cells and the body begins to fight off the Beast.
In other cases, your body chemistry is different than mine and may lack some sort protein/? or what ever. So in this case, the communication is lost like a drop signal from your cell phone. What you need is another Cell Tower to transmit that signal. That is where CTLA-4 coming in to play. It builds the tower to help with the communication. I may be over simplifying the biochemistry but I am not in the lab to do the right experiments. So, I have to take an educated guess.
So, I did a little online research
As you can see, Dr. Kirkwood and I wanted to induce tumor regression by using my own immune system. If we could get my immune system to recognize the tumors as foreign, then we might have a fighting chance.
So we decide to try the CTLA-4 Therapy, Antitumor response with prolonged time to progression has been seen in patients with melanoma who have received either of the CTLA-4 antibodies and durable antitumor responses have been observed with ipilimumab in patients with melanoma ovarian cancer, prostate cancer, and renal cell carcinoma It has been seen, antitumor responses may be characterized by short-term progression followed by delayed regression.
An important, possibly unique, clinical characteristic of anti-CTLA-4 antibodies is that the duration of clinical response -- and even stable disease -- is often quite prolonged.
This is what I believe is going on in my case but I have no proof.
So lets combine the two clinical trials and that was done by Dr. Rosenberg at the National Cancer Institute. This did not yield the Better outcome.
I think it is because doing the therapy at the same time my be jamming the signals or changing the micro-chemical in the cell and surrounding environment so that it is not inductive to passing on the communication cell to cell.. protein to protein .
But since there was a lag between my two therapies, My cells began to communicate and jump started my immune system. Do I think is a one off, NO!!!!! I have been in touch with another patient that did IL-2 then, CTLA-4 and got the same response. The trick is to get the timing and the dose right!!!!!
So I took it apon myself to to a little research and this is what I came up with. I believe it all makes sense. I still may be over simplifying the actual process but I am not a biochemist. So Here goes:
Dendritic cells (DCs) are immune cells and form part of the our immune system. Their main function is to process antigen material and present it on the surface to other cells of the immune system, thus functioning as Antigen-Presenting Cells (APC).
The dendritic cells are constantly in communication with other cells in the body. This communication can take the form of direct cell-to-cell contact based on the interaction of cell-surface proteins. An example of this includes the interaction of the receptor B7 of the dendritic cell with CD28 present on the lymphocyte. However, the cell-cell interaction can also take place at a distance via cytokines. These components of the immune system communicate with one another by exchanging chemical messengers. These proteins are secreted by cells and act on other cells to coordinate an appropriate immune response.
Cytokines include a diverse assortment of interleukins, interferons, and growth factors.One cytokine, interleukin 2 (IL-2), triggers the immune system to produce T cells. IL-2’s immunity-boosting properties have traditionally made it a promising treatment for several illnesses which include Hepatitis C and Melanoma.
There are several steps to activation of the immune system against a foreign molecule. The T cell receptor must first interact with the MHC molecule. The T cell receptor or TCR is a molecule found on the surface of T lymphocytes (or T cells) that is, in general, responsible for recognizing antigens bound to Major Histocompatibility Complex (MHC) molecules. MHC the most gene-dense region of the Human genome and plays an important role in the immune system, autoimmunity.
This first interaction involves the CD4 or CD8 proteins which form a complex with the CD3 protein to bind to the MHC molecule of the (APC). Antigen-presenting cell This is also called "Signal 1" and its main purpose is T cell activation.
However, this is insufficient for producing a T cell response by itself. In fact, lack of further stimulatory signals sends the T cell into anergy. Anergy is a term in immunobiology that describes a lack of reaction by the body's defense mechanisms to foreign substances.
The Second costimulatory signal necessary to continue the immune response can come from B7-CD28 and CD40-CD40L interactions. The primary role of the B7 proteins is to give a second signal to the T cell. The B7 protein/receptor is present on the Antigen-presenting cell and is able to interact with the CD28 receptor on the T cell surface; this is also known as "Signal 2". There are other activation signals which play a role in immune responses.
On these T cells there is are family receptors whose job is downregulate the T cell activation so the immune system maintains metabolic equilibrium so the immune system doesn’t start an autoimmune response and cause it to attack itself. One of these receptors is Cytotoxic T lymphocyte-associated antigen (CTLA4).
It was hypothesized back in the 1980’s that if you replaced the CTLA4 with Anti-CTLA4 that it might block the B7 receptor causing an enhancement of the T-cell activation, leading to a more robust antitumor immune response.
It was shown in mice with a disrupted CTLA-4 genes that their immune response ran unabated causing autoimmunity which was fatal.
It was also shown that the anti-CTLA-4 antibodies had a greater affinity to CTLA-4 than the B7 receptor. So by doing the CTLA-4 Therapy, it allowed signal 1 to become active.
So in the presents of the CTLA-4 antibody Therapy, I my case, we may have extended the antitumor response of the T-cells. This left Signal 1 active.
We then, hit the immune system with High dose of IL-2. This must have stimulated the cell to cell communication (Signal 2) causing the immune response to kick in against the foreign molecule (The Tumor)
AS YOU GUESSED IT, I MUST HAVE JUMP STARTED MY IMMUNE SYSTEM!!!!!!
Just don't know how long it will last.
AS you can see , I am passionate about finding a cure. My Life depends on it. So if I can pass along information that may help others, I do. And that is why I am asking for your Permission on post this information.
I hope to here from you soon
Thanks for your time and Keep the good research coming!!!!
Sincerely,
James M. Breitfeller
The information contained is intended for the confidential use of the intended recipient(s) only and may be legally privileged. If it has been received other than by an intended recipient, it may not be reviewed, disseminated, distributed, copied or retained. Therefore, please notify the sender immediately and delete the original message and all copies.
Thank you
James M. Breitfeller
Understanding Cancer Series: The Immune System (National Cancer Institute) Melanoma...Jim Breitfeller
This is a must read. There is 38 sides. If you read them, the papers that I have been posting will start to make sense. It could save your life or your love ones life.
http://www.cancer.gov/cancertopics/understandingcancer/immunesystem
immune system
I promise you will not be disappointed.
Jimmy B
http://www.cancer.gov/cancertopics/understandingcancer/immunesystem
immune system
I promise you will not be disappointed.
Jimmy B
Financial Help for lost income... if you got Melanoma (SS Disability) ..Jim Breitfeller
If you Have Melanoma and you are out of work, you can file for SS disability. It may help pay some bills. Every little bit helps. You need to get your doctors involved.
Here is the criteria.
13.03 Skin.
A. Sarcoma or carcinoma with metastases to or beyond the regional lymph nodes.
OR
B. Melanoma, with either 1 or 2:
1. Recurrent after wide excision (except an additional primary melanoma at a different site, which is not considered to be recurrent disease).
2. Palpable nodal metastases or metastases to adjacent skin (satellite lesions) or elsewhere.
It has help me stay afloat.
Here is the criteria.
13.03 Skin.
A. Sarcoma or carcinoma with metastases to or beyond the regional lymph nodes.
OR
B. Melanoma, with either 1 or 2:
1. Recurrent after wide excision (except an additional primary melanoma at a different site, which is not considered to be recurrent disease).
2. Palpable nodal metastases or metastases to adjacent skin (satellite lesions) or elsewhere.
It has help me stay afloat.
Tuesday, January 13, 2009
am Very Sadden,we lost another true Warrior Today
Please, If you get a chance, go to Kyle's site and pay your respect to the family.
He is finally out of pain, and in the Lord's Hand.
Thanks
cp: kylefightsback
www.carepages.com
He is finally out of pain, and in the Lord's Hand.
Thanks
cp: kylefightsback
www.carepages.com
The Bottom Line!!!!! Melanoma Jim Breitfeller
The Bottom Line, don't worry if you don't understand it. I will have to read it 5 to 10 times before I just start to understand 1/20th of what they said. So, If you want skip to the section at the bottom of the article call TARGETED MOLECULAR THERAPY and the Conclusion.
What to take away from this article.
1) The researchers are getting closer to understanding how things work with melanoma.
2) your blood chemistry and your tumor type will play a very important roll in what path of therapy will work for you.
3) This is so new, that your Oncologist may not even know about this.This is Cutting Edge Technology at it's best.
4)The BEST melanoma patient is an ACTIVE PARTICIPANT in his
or her treatment (not a PASSIVE RECEPIENT)
5) Live today as if there was no Tomorrow.
Jimmy B
What to take away from this article.
1) The researchers are getting closer to understanding how things work with melanoma.
2) your blood chemistry and your tumor type will play a very important roll in what path of therapy will work for you.
3) This is so new, that your Oncologist may not even know about this.This is Cutting Edge Technology at it's best.
4)The BEST melanoma patient is an ACTIVE PARTICIPANT in his
or her treatment (not a PASSIVE RECEPIENT)
5) Live today as if there was no Tomorrow.
Jimmy B
Malignant melanoma in the 21st century: the emerging molecular landscape.!!!! Jim Breitfeller
I hit The Mother Load!!!!!!!!!!!!!!!!!!
Sekulic A, Haluska P Jr, Miller AJ, Genebriera De Lamo J, Ejadi S, Pulido JS, Salomao DR, Thorland EC, Vile RG, Swanson DL, Pockaj BA, Laman SD, Pittelkow MR, Markovic SN; Melanoma Study Group of Mayo Clinic Cancer Center.
Collaborators (79)
Allred JB, Anastasiadis PZ, Baratz KH, Bite U, Bradley EA, Bradshaw RK, Cameron JD, Clay RP, Connolly SM, Copland JA 3rd, Creagan ET, Croghan GA, Davis MD, Dietz AB, Donohue JH, Erickson LA, Farley DR, Flotte TJ, Galanis E, Garces YI, Garrity JA, Gastineau DA, Gostout BS, Grant CS, Hand JL, Ingle JN, Kalaaji AN, Kasperbauer JL, Kaur JS, Kottschade LA, Keeling J, Laman SD, Lim KK, Lindor NM, Loprinzi CL, Lowe V, Lustgarten J, Maguire LJ, Maples WJ, Markovic SN, McCannel CA, McDonald ES, McWilliams RR, Milburn JM, Moran SL, Nevala WK, Olsen KD, Otley CC, Pardanani A, Perdikis G, Pittelkow MR, Pitts JM, Pockaj BA, Podratz KC, Pulido JS, Rock MG, Roenigk RK, Salassa JR, Salomao DR, Schild SE, Sekulic A, Shives TC, Sim FH, Snow JL, Stanhope CR, Su WP, Suman VJ, Swanson DL, Tan W, TerKonda SP, Tran NV, Vachon CM, Vile RG, Vuk-Pavlovic S, Waldorf JC, Weenig RH, Wilcox RA, Wilson TO, Wiseman GA.
Department of Dermatology, Mayo Clinic, Scottsdale, AZ 85259, USA. sekulic.aleksandar@mayo.edu
Malignant melanoma presents a substantial clinical challenge. Current diagnostic methods are limited in their ability to diagnose early disease and accurately predict individual risk of disease progression and outcome. The lack of adequate approaches to properly define disease subgroups precludes rational treatment design and selection. Better tools are urgently needed to provide more accurate and personalized melanoma patient management. Recent progress in the understanding of the molecular aberrations that underlie melanoma oncogenesis will likely advance the diagnosis, prognosis, and treatment of melanoma. The emerging pattern of molecular complexity in melanoma tumors mirrors the clinical diversity of the disease and highlights the notion that melanoma, like other cancers, is not a single disease but a heterogeneous group of disorders that arise from complex molecular changes. Understanding of molecular aberrations involving important cellular processes, such as cellular signaling networks, cell cycle regulation, and cell death, will be essential for better diagnosis, accurate assessment of prognosis, and rational design of effective therapeutics. Defining an individual patient's unique tumor characteristics may lead to personalized prediction of outcomes and selection of therapy. We review the emerging molecular landscape of melanoma and its implications for better management of patients with melanoma.
PMID: 18613999 [PubMed - indexed for MEDLINE]
Malignant Melanoma in the 21st Century:
The Emerging Molecular Landscape
eAPPENDIX. Molecularly Targeted Therapeutics in Melanoma
eAPPENDIX. Molecularly Targeted Therapeutics in Melanoma
Have fun reading. You are going to need a medical dictionary!!!!!!
Take little bites.
Jimmy B
Sekulic A, Haluska P Jr, Miller AJ, Genebriera De Lamo J, Ejadi S, Pulido JS, Salomao DR, Thorland EC, Vile RG, Swanson DL, Pockaj BA, Laman SD, Pittelkow MR, Markovic SN; Melanoma Study Group of Mayo Clinic Cancer Center.
Collaborators (79)
Allred JB, Anastasiadis PZ, Baratz KH, Bite U, Bradley EA, Bradshaw RK, Cameron JD, Clay RP, Connolly SM, Copland JA 3rd, Creagan ET, Croghan GA, Davis MD, Dietz AB, Donohue JH, Erickson LA, Farley DR, Flotte TJ, Galanis E, Garces YI, Garrity JA, Gastineau DA, Gostout BS, Grant CS, Hand JL, Ingle JN, Kalaaji AN, Kasperbauer JL, Kaur JS, Kottschade LA, Keeling J, Laman SD, Lim KK, Lindor NM, Loprinzi CL, Lowe V, Lustgarten J, Maguire LJ, Maples WJ, Markovic SN, McCannel CA, McDonald ES, McWilliams RR, Milburn JM, Moran SL, Nevala WK, Olsen KD, Otley CC, Pardanani A, Perdikis G, Pittelkow MR, Pitts JM, Pockaj BA, Podratz KC, Pulido JS, Rock MG, Roenigk RK, Salassa JR, Salomao DR, Schild SE, Sekulic A, Shives TC, Sim FH, Snow JL, Stanhope CR, Su WP, Suman VJ, Swanson DL, Tan W, TerKonda SP, Tran NV, Vachon CM, Vile RG, Vuk-Pavlovic S, Waldorf JC, Weenig RH, Wilcox RA, Wilson TO, Wiseman GA.
Department of Dermatology, Mayo Clinic, Scottsdale, AZ 85259, USA. sekulic.aleksandar@mayo.edu
Malignant melanoma presents a substantial clinical challenge. Current diagnostic methods are limited in their ability to diagnose early disease and accurately predict individual risk of disease progression and outcome. The lack of adequate approaches to properly define disease subgroups precludes rational treatment design and selection. Better tools are urgently needed to provide more accurate and personalized melanoma patient management. Recent progress in the understanding of the molecular aberrations that underlie melanoma oncogenesis will likely advance the diagnosis, prognosis, and treatment of melanoma. The emerging pattern of molecular complexity in melanoma tumors mirrors the clinical diversity of the disease and highlights the notion that melanoma, like other cancers, is not a single disease but a heterogeneous group of disorders that arise from complex molecular changes. Understanding of molecular aberrations involving important cellular processes, such as cellular signaling networks, cell cycle regulation, and cell death, will be essential for better diagnosis, accurate assessment of prognosis, and rational design of effective therapeutics. Defining an individual patient's unique tumor characteristics may lead to personalized prediction of outcomes and selection of therapy. We review the emerging molecular landscape of melanoma and its implications for better management of patients with melanoma.
PMID: 18613999 [PubMed - indexed for MEDLINE]
Malignant Melanoma in the 21st Century:
The Emerging Molecular Landscape
eAPPENDIX. Molecularly Targeted Therapeutics in Melanoma
eAPPENDIX. Molecularly Targeted Therapeutics in Melanoma
Have fun reading. You are going to need a medical dictionary!!!!!!
Take little bites.
Jimmy B
Link Between T Cell Response To New Melanoma Antigen And Relapse-Free Survival Melanoma.. Jim Breitfeller
Main Category: Cancer / Oncology
Also Included In: Dermatology; Immune System / Vaccines
Article Date: 21 Oct 2008 - 3:00 PDT
Adoptive Cell Transfer Therapy!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
DR. Steven A. Rosenberg at The National Cancer Institute
Melanoma patients infused with a special type of tumor-fighting T cell are more likely to survive without relapse, suggests a new study by researchers in France. Their report was published online on October 20 in the Journal of Experimental Medicine.
One treatment option for patients with late-stage melanoma involves removing natural cancer-fighting T cells from the tumor, expanding their numbers in culture dishes, and then re-infusing them into the patient. This strategy - called adoptive immunotherapy - causes tumor regression in about half the patients treated, some of whom survive for decades without relapse.
The French team, lead by Dr. Nathalie Labarriere, studied the infused cells from 30 stage III melanoma patients who had received adoptive immunotherapy between 1994 and 1998. Among the cells taken from a patient who has remained tumor-free for more than a decade, they found naturally-arising T cells that recognized a new protein, which they dubbed "meloe-1." Meloe-1, the group found, is highly expressed in melanoma cells but not in normal skin cells or in other types of cancer.
When they looked at the transferred cells from the other patients, they found meloe-1-specific T cells in 5 of the 9 patients who remained relapse-free, but in none of the 21 patients who relapsed. The association of meloe-1-specific T cells with relapse-free survival suggests that amplifying these T cells in culture may be one way to improve the efficacy of adoptive immunotherapy. The team has also succeeded in finding meloe-1 T cells in patients' circulating blood - a much more accessible source than tumor tissue.
----------------------------
Article adapted by Medical News Today from original press release.
----------------------------
That is a 50%+ complete Response WE are half way there!!!!!!!!!
It can also be done by CTLA-4 Blockage to stimulate the T CELL.
That is what Happen to me. And then we did the IL-2 for the cell to cell
commumination.
WE are getting Closer to the CCCCC UUUUU RRRRR EEEEE!!!!!
Five-year View
"Several advances are being made in the field of immunological and targeted therapies. In our opinion, the agents of most interest in the next 5 years would be the anti-CTLA-4 antibodies and angiogenesis inhibitors combined with cytotoxic therapy. While most of these novel agents may not have significant single-agent activity, combining them with systemic chemotherapy might be the key to clinical success in melanoma. Ultimately, rational combination therapeutics of multimodal targeting agents (immunotherapeutics, cytotoxic therapy, angiogenesis inhibitors) will likely lead to substantive clinical benefit in this patient population. Our hope is that this massive clinical research endeavor will soon yield the long sought after improvement in the overall survival of patients with advanced melanoma."
Dr.Svetomir N Markovic
Rajini Katipamula; Svetomir N Markovic
Expert Rev Anticancer Ther. 2008;8(4):553-560.
Posted 07/09/2008
Svetomir N. Markovic, M.D.
Location: Minnesota
Education
Fellowship – Department of Internal Medicine; Division of Hematology, Department of Oncology
Mayo Graduate School of Medicine
Residency – Internal Medicine
Mayo Graduate School of Medicine
M.D.
Medical College of Pennsylvania
Fellowship – Part-time Post-doctoral; Microbiology/Immunology, Pharmacology
Medical College of Pennsylvania
Ph.D. – Department of Microbiology/Immunology
Medical College of Pennsylvania
Summary
Translational immunotherapeutics of cancer focused on malignant melanoma and non-Hodgkin's lymphoma. This work includes development and clinical testing of: cancer vaccines; immune boosting agents; novel agents that reconstitute immunity in patients with cancer; and combination therapy directed at enhancing anti-tumor immune responses.
Also Included In: Dermatology; Immune System / Vaccines
Article Date: 21 Oct 2008 - 3:00 PDT
Adoptive Cell Transfer Therapy!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
DR. Steven A. Rosenberg at The National Cancer Institute
Melanoma patients infused with a special type of tumor-fighting T cell are more likely to survive without relapse, suggests a new study by researchers in France. Their report was published online on October 20 in the Journal of Experimental Medicine.
One treatment option for patients with late-stage melanoma involves removing natural cancer-fighting T cells from the tumor, expanding their numbers in culture dishes, and then re-infusing them into the patient. This strategy - called adoptive immunotherapy - causes tumor regression in about half the patients treated, some of whom survive for decades without relapse.
The French team, lead by Dr. Nathalie Labarriere, studied the infused cells from 30 stage III melanoma patients who had received adoptive immunotherapy between 1994 and 1998. Among the cells taken from a patient who has remained tumor-free for more than a decade, they found naturally-arising T cells that recognized a new protein, which they dubbed "meloe-1." Meloe-1, the group found, is highly expressed in melanoma cells but not in normal skin cells or in other types of cancer.
When they looked at the transferred cells from the other patients, they found meloe-1-specific T cells in 5 of the 9 patients who remained relapse-free, but in none of the 21 patients who relapsed. The association of meloe-1-specific T cells with relapse-free survival suggests that amplifying these T cells in culture may be one way to improve the efficacy of adoptive immunotherapy. The team has also succeeded in finding meloe-1 T cells in patients' circulating blood - a much more accessible source than tumor tissue.
----------------------------
Article adapted by Medical News Today from original press release.
----------------------------
That is a 50%+ complete Response WE are half way there!!!!!!!!!
It can also be done by CTLA-4 Blockage to stimulate the T CELL.
That is what Happen to me. And then we did the IL-2 for the cell to cell
commumination.
WE are getting Closer to the CCCCC UUUUU RRRRR EEEEE!!!!!
Five-year View
"Several advances are being made in the field of immunological and targeted therapies. In our opinion, the agents of most interest in the next 5 years would be the anti-CTLA-4 antibodies and angiogenesis inhibitors combined with cytotoxic therapy. While most of these novel agents may not have significant single-agent activity, combining them with systemic chemotherapy might be the key to clinical success in melanoma. Ultimately, rational combination therapeutics of multimodal targeting agents (immunotherapeutics, cytotoxic therapy, angiogenesis inhibitors) will likely lead to substantive clinical benefit in this patient population. Our hope is that this massive clinical research endeavor will soon yield the long sought after improvement in the overall survival of patients with advanced melanoma."
Dr.Svetomir N Markovic
Rajini Katipamula; Svetomir N Markovic
Expert Rev Anticancer Ther. 2008;8(4):553-560.
Posted 07/09/2008
Svetomir N. Markovic, M.D.
Location: Minnesota
Education
Fellowship – Department of Internal Medicine; Division of Hematology, Department of Oncology
Mayo Graduate School of Medicine
Residency – Internal Medicine
Mayo Graduate School of Medicine
M.D.
Medical College of Pennsylvania
Fellowship – Part-time Post-doctoral; Microbiology/Immunology, Pharmacology
Medical College of Pennsylvania
Ph.D. – Department of Microbiology/Immunology
Medical College of Pennsylvania
Summary
Translational immunotherapeutics of cancer focused on malignant melanoma and non-Hodgkin's lymphoma. This work includes development and clinical testing of: cancer vaccines; immune boosting agents; novel agents that reconstitute immunity in patients with cancer; and combination therapy directed at enhancing anti-tumor immune responses.
Labels:
CTLA-4,
Dr. Labarriere,
Gene Therapy,
Melanoma,
Meloe-1,
Rosenberg
New Data On Melanoma Treatment Dicarbazine and or Temozolomide 2008 Melamona ..Jim Breitfeller
Main Category: Cancer / Oncology
Also Included In: Genetics; Dermatology; Clinical Trials / Drug Trials
Article Date: 16 Sep 2008 - 3:00 PDT
People who carry a particular genetic variant are at significantly increased risk of developing malignant melanoma, new research shows.
Melanomas are known to be caused by exposure to the ultraviolet light in sunlight, but the precise mechanisms involved are complex. In a presentation at the 33rd Congress of the European Society for Medical Oncology (ESMO) in Stockholm, Portuguese researchers showed that variations in a gene known as cyclin D1 also increase susceptibility to the disease.
This gene plays a key role in regulating the cell cycle, the intricate molecular process by which cells divide and replicate. Alterations in its activity are known to be associated with the development of several human cancers, including melanoma.
PhD student Raquel Catarino from the Portuguese Institute of Oncology in Porto and colleagues studied a particular variant of the gene in the blood of 1,053 individuals, including 161 cases with melanoma and 892 healthy individuals. Their analysis showed that individuals carrying two copies of the variant were 80% more likely to develop melanoma.
"Our study demonstrates that cyclin D1 polymorphism is associated with a higher risk of melanoma development, indicating that this genetic variation may confer growth advantage to cancer cells. Our results indicate that the proportion of melanoma cases attributable to this genetic alteration is 14%."
Other research groups have identified other genes that are implicated in susceptibility to melanoma. "We think that once the genetic factors involved in melanoma oncogenesis are identified and their importance established and validated, the individual's genetic profile could help clinical decisions, including disease screening and selection of higher-risk individuals," Dr. Catarino said.
In another presentation, Prof. Poulam Patel from Nottingham University in the UK reported the final results from a large randomized phase III study in 859 patients with stage IV melanoma. The clinical trial, coordinated by the EORTC Melanoma Study Group, involving 92 institutions in Europe, the US and Latin America, is the largest of its kind in this group of patients.
In the trial, chemotherapy-naive patients with stage IV disease were treated with either dicarbazine 1000 mg/m2 IV every 21 days (the current standard treatment) or temozolomide 150 mg/m2 orally on days 1 - repeated every 14 days. "Temozolomide is an oral chemotherapy which has activity against melanoma and this regimen is a dose-intense way of delivering the treatment in the hope of delivering more active drug and more effectively," Prof. Patel said. "The study showed that although there were small differences in the response rate and side effects, there was no difference in the overall survival or progression-free survival."
"We continue to look for new treatments that will show benefit when tested in a large phase III study," he said.
----------------------------
Article adapted by Medical News Today from original press release.
----------------------------
Well I tried Dicarbazine and had no clinical response!!!!!!!!
Jimmy B
Also Included In: Genetics; Dermatology; Clinical Trials / Drug Trials
Article Date: 16 Sep 2008 - 3:00 PDT
People who carry a particular genetic variant are at significantly increased risk of developing malignant melanoma, new research shows.
Melanomas are known to be caused by exposure to the ultraviolet light in sunlight, but the precise mechanisms involved are complex. In a presentation at the 33rd Congress of the European Society for Medical Oncology (ESMO) in Stockholm, Portuguese researchers showed that variations in a gene known as cyclin D1 also increase susceptibility to the disease.
This gene plays a key role in regulating the cell cycle, the intricate molecular process by which cells divide and replicate. Alterations in its activity are known to be associated with the development of several human cancers, including melanoma.
PhD student Raquel Catarino from the Portuguese Institute of Oncology in Porto and colleagues studied a particular variant of the gene in the blood of 1,053 individuals, including 161 cases with melanoma and 892 healthy individuals. Their analysis showed that individuals carrying two copies of the variant were 80% more likely to develop melanoma.
"Our study demonstrates that cyclin D1 polymorphism is associated with a higher risk of melanoma development, indicating that this genetic variation may confer growth advantage to cancer cells. Our results indicate that the proportion of melanoma cases attributable to this genetic alteration is 14%."
Other research groups have identified other genes that are implicated in susceptibility to melanoma. "We think that once the genetic factors involved in melanoma oncogenesis are identified and their importance established and validated, the individual's genetic profile could help clinical decisions, including disease screening and selection of higher-risk individuals," Dr. Catarino said.
In another presentation, Prof. Poulam Patel from Nottingham University in the UK reported the final results from a large randomized phase III study in 859 patients with stage IV melanoma. The clinical trial, coordinated by the EORTC Melanoma Study Group, involving 92 institutions in Europe, the US and Latin America, is the largest of its kind in this group of patients.
In the trial, chemotherapy-naive patients with stage IV disease were treated with either dicarbazine 1000 mg/m2 IV every 21 days (the current standard treatment) or temozolomide 150 mg/m2 orally on days 1 - repeated every 14 days. "Temozolomide is an oral chemotherapy which has activity against melanoma and this regimen is a dose-intense way of delivering the treatment in the hope of delivering more active drug and more effectively," Prof. Patel said. "The study showed that although there were small differences in the response rate and side effects, there was no difference in the overall survival or progression-free survival."
"We continue to look for new treatments that will show benefit when tested in a large phase III study," he said.
----------------------------
Article adapted by Medical News Today from original press release.
----------------------------
Well I tried Dicarbazine and had no clinical response!!!!!!!!
Jimmy B
Monday, January 12, 2009
Impact of false-negative sentinel lymph node biopsy on survival in patients with cutaneous melanoma. Jim Breitfeller
Ann Surg Oncol. 2007 Sep;14(9):2662-7. Epub 2007 Jun 28
National Cancer Institute, Via M. Semmola, 80131, Naples, Italy. corracara@fastwebnet.it
BACKGROUND AND OBJECTIVES: Sentinel lymph node biopsy is widely accepted as standard care in melanoma despite lack of pertinent randomized trials results. A possible pitfall of this procedure is the inaccurate identification of the sentinel lymph node leading to biopsy and analysis of a nonsentinel node. Such a technical failure may yield a different prognosis. The purpose of this study is to analyze the incidence of false negativity and its impact on clinical outcome and to try to understand its causes. METHODS: The Melanoma Data Base at National Cancer Institute of Naples was analyzed comparing results between false-negative and tumor-positive sentinel node patients focusing on overall survival and prognostic factors influencing the clinical outcome.
RESULTS: One hundred fifty-one cases were diagnosed to be tumor-positive after sentinel lymph node biopsy and were subjected to complete lymph node dissection. Thirty-four (18.4%)patients with tumor-negative sentinel node subsequently developed lymph node metastases in the basin site of the sentinel procedure. With a median follow-up of 42.8 months the 5-year overall survival was 48.4% and 66.3% for false-negative and tumor-positive group respectively with significant statistical differences (P < .03). CONCLUSIONS: The sensitivity of sentinel lymph node biopsy was 81.6%, and a regional nodal basin recurrence after negative-sentinel node biopsy means a worse prognosis, compared with patients submitted to complete lymph node dissection after a positive sentinel biopsy. The evidence of higher number of tumor-positive nodes after delayed lymphadenectomy in false-negative group compared with tumor-positive sentinel node cases, confirmed the importance of an early staging of lymph nodal involvement. Further data will better clarify the role of prognostic factors to identify cases with a more aggressive biological behavior of the disease.
See, I had a False negative on my SLN Biopsy.
Eeny, meeny, miny, moe catch a MELANOMA cell by it's toe!!!!!!
Will the real Sentinel Lymph Node Please Lite up!!!!!!!!
Jimmy B
National Cancer Institute, Via M. Semmola, 80131, Naples, Italy. corracara@fastwebnet.it
BACKGROUND AND OBJECTIVES: Sentinel lymph node biopsy is widely accepted as standard care in melanoma despite lack of pertinent randomized trials results. A possible pitfall of this procedure is the inaccurate identification of the sentinel lymph node leading to biopsy and analysis of a nonsentinel node. Such a technical failure may yield a different prognosis. The purpose of this study is to analyze the incidence of false negativity and its impact on clinical outcome and to try to understand its causes. METHODS: The Melanoma Data Base at National Cancer Institute of Naples was analyzed comparing results between false-negative and tumor-positive sentinel node patients focusing on overall survival and prognostic factors influencing the clinical outcome.
RESULTS: One hundred fifty-one cases were diagnosed to be tumor-positive after sentinel lymph node biopsy and were subjected to complete lymph node dissection. Thirty-four (18.4%)patients with tumor-negative sentinel node subsequently developed lymph node metastases in the basin site of the sentinel procedure. With a median follow-up of 42.8 months the 5-year overall survival was 48.4% and 66.3% for false-negative and tumor-positive group respectively with significant statistical differences (P < .03). CONCLUSIONS: The sensitivity of sentinel lymph node biopsy was 81.6%, and a regional nodal basin recurrence after negative-sentinel node biopsy means a worse prognosis, compared with patients submitted to complete lymph node dissection after a positive sentinel biopsy. The evidence of higher number of tumor-positive nodes after delayed lymphadenectomy in false-negative group compared with tumor-positive sentinel node cases, confirmed the importance of an early staging of lymph nodal involvement. Further data will better clarify the role of prognostic factors to identify cases with a more aggressive biological behavior of the disease.
See, I had a False negative on my SLN Biopsy.
Eeny, meeny, miny, moe catch a MELANOMA cell by it's toe!!!!!!
Will the real Sentinel Lymph Node Please Lite up!!!!!!!!
Jimmy B
Free Transport for you and your caregiver (Wife) !!!! Melanoma jim Breitfeller
Free Transport for you and your caregiver (Wife) to get the medical care you need.
Angel Flight: 1-800-549-9980 (Paula or Diane/contacts)
angel flight Northeast
You have to earn your wings!!!!!!
I just found out about two families that could have use this information.
I am SORRY THAT I DID NOT POST THIS SOONER.
See, I myself had used it in the begining because the it was adding up fast to get to all those appointment and second opinions Consults.
Please bookmark this site because you never know if you will need it.
Also, for those in the other parts of the country, I believe that they too have this care and Paula and Diane could probably get the phone number for you.
See, one wife was not allow to go with her husband because the insurance would not cover it.
NOW THAT IS JUST NOT RIGHT!!!!!!!
Jimmy B
Angel Flight: 1-800-549-9980 (Paula or Diane/contacts)
angel flight Northeast
You have to earn your wings!!!!!!
I just found out about two families that could have use this information.
I am SORRY THAT I DID NOT POST THIS SOONER.
See, I myself had used it in the begining because the it was adding up fast to get to all those appointment and second opinions Consults.
Please bookmark this site because you never know if you will need it.
Also, for those in the other parts of the country, I believe that they too have this care and Paula and Diane could probably get the phone number for you.
See, one wife was not allow to go with her husband because the insurance would not cover it.
NOW THAT IS JUST NOT RIGHT!!!!!!!
Jimmy B
Sunday, January 11, 2009
One of our family Member asked about C kit testing!!!! jim Breitfeller
This is another important test for patients with c-kit mutation positive melanoma
This from the University of Utah Pathology Department
Targeted Cancer Therapy in Metastatic Malignant Melanoma
last modified 2008-03-06 18:29 — by Dave
Problem:
"When it comes to cancer patients with metastatic melanoma, there is little that can be done on their behalf. But for a few patients, studies are currently underway that could drastically improve their likelihood of survival."
How it all started:
"The research began a few years ago when scientists in the Division of Anatomic Pathology in the Department of Pathology at the University of Utah were studying gastrointestinal stromal tumors (GISTs) with mutations in the c-kit gene. KIT, the protein coded for by the c-kit gene, normally activates cellular signals which tell the cell to grow and divide. In most normal circumstances, the c-kit gene is turned off and KIT is silent. However, it was discovered that GISTs have mutations in the c-kit gene which result in a KIT protein that is activated, or in other words is always turned on. Activation of the KIT protein causes a constant signal for the GIST tumor cells to grow. It has been discovered that GISTs, a tumor which previously had been therapeutically resistant, can be controlled by use of a new drug called Gleevec. Gleevec works as a tyrosine kinase inhibitor that binds to the active site of the mutationally active tyrosine kinase KIT and inhibits the protein. Inhibition of KIT stops cellular signaling and turns off tumor growth. There are several different types of c-kit activating mutations occurring in GISTs and some are more responsive to Gleevec than others. To aid in the treatment of GIST patients, it is now possible, through the research efforts in Anatomic Pathology, to determine the type of c-kit mutation in GISTs."
The rest can be found at their website:
http://www.path.utah.edu/news/targeted-cancer-therapy
targeted-cancer-therapy
This is why it is so important to know your Blood chemistry!!!!!!!! It will help you find the right path to the right therapy.
Patty, thank you for sharing. This is how we all will become self-educated in Melanoma Therapy.
Jimmy B
This from the University of Utah Pathology Department
Targeted Cancer Therapy in Metastatic Malignant Melanoma
last modified 2008-03-06 18:29 — by Dave
Problem:
"When it comes to cancer patients with metastatic melanoma, there is little that can be done on their behalf. But for a few patients, studies are currently underway that could drastically improve their likelihood of survival."
How it all started:
"The research began a few years ago when scientists in the Division of Anatomic Pathology in the Department of Pathology at the University of Utah were studying gastrointestinal stromal tumors (GISTs) with mutations in the c-kit gene. KIT, the protein coded for by the c-kit gene, normally activates cellular signals which tell the cell to grow and divide. In most normal circumstances, the c-kit gene is turned off and KIT is silent. However, it was discovered that GISTs have mutations in the c-kit gene which result in a KIT protein that is activated, or in other words is always turned on. Activation of the KIT protein causes a constant signal for the GIST tumor cells to grow. It has been discovered that GISTs, a tumor which previously had been therapeutically resistant, can be controlled by use of a new drug called Gleevec. Gleevec works as a tyrosine kinase inhibitor that binds to the active site of the mutationally active tyrosine kinase KIT and inhibits the protein. Inhibition of KIT stops cellular signaling and turns off tumor growth. There are several different types of c-kit activating mutations occurring in GISTs and some are more responsive to Gleevec than others. To aid in the treatment of GIST patients, it is now possible, through the research efforts in Anatomic Pathology, to determine the type of c-kit mutation in GISTs."
The rest can be found at their website:
http://www.path.utah.edu/news/targeted-cancer-therapy
targeted-cancer-therapy
This is why it is so important to know your Blood chemistry!!!!!!!! It will help you find the right path to the right therapy.
Patty, thank you for sharing. This is how we all will become self-educated in Melanoma Therapy.
Jimmy B
HLA-2a Definition Please add it to the file!!!!!!! Jim Breitfeller
There is No questions here are dumb. This is a learning Experience. I am learning all the time because Knowledge will take me to the right path of this journey.
HLA-A2 is a blood test that you can have your PCP fill out a script for.
A tissue type test is a blood test that measures substances called antigens on the surface of body cells and tissues. Checking the antigens can tell if donor tissue is safe (compatible) for transplant to another person. This test may also be called HLA typing. Antigens can tell the difference between normal body tissue or foreign tissue (for example, tissue from another person's body). Tissue type helps find the best match for tissues or blood cells (such as platelets). In some cases, a tissue type test may be done to see whether a person has a chance for developing certain diseases that cause the body to attack its own cells, such as autoimmune diseases.
A special pattern of antigens (called tissue type) is present on each person's cells and tissues. Half of each person's antigens come from (inherited) the mother and half from the father. Identical twins have the same pattern, but everyone else has his or her own special pattern. Brothers and sisters have a 1-in-4 chance of having an identical match. Each person's antigen pattern can be "fingerprinted" through a tissue type test.
HLA-A2 is a blood test that you can have your PCP fill out a script for.
A tissue type test is a blood test that measures substances called antigens on the surface of body cells and tissues. Checking the antigens can tell if donor tissue is safe (compatible) for transplant to another person. This test may also be called HLA typing. Antigens can tell the difference between normal body tissue or foreign tissue (for example, tissue from another person's body). Tissue type helps find the best match for tissues or blood cells (such as platelets). In some cases, a tissue type test may be done to see whether a person has a chance for developing certain diseases that cause the body to attack its own cells, such as autoimmune diseases.
A special pattern of antigens (called tissue type) is present on each person's cells and tissues. Half of each person's antigens come from (inherited) the mother and half from the father. Identical twins have the same pattern, but everyone else has his or her own special pattern. Brothers and sisters have a 1-in-4 chance of having an identical match. Each person's antigen pattern can be "fingerprinted" through a tissue type test.
DATE 12-09-2008 I contacted Dr. Cassian Yee, M.D. Jim Breitfeller
Dr. Cassian Yee, M.D. associate member, clinical research division, Fred Hutchinson Cancer Research Center, Seattle
Dr. Yee could you email a copy of this paper.
I had e-mail Dr. Yee on 12-9-2008 for his paper
I am a cancer patient (50 yrs. old) under the care of Dr. John Kirkwood at the Hillman Cancer Center at the University of Pittsburgh. I have gone through a wide incision, lymph nodes removal, Interferon therapy, and Dicarbazine therapy and CTLA-4 without success. I am presently washed out an IL-2 clinical trial that started in November 1, 2006. On the fourth cycle I had a heart attack and the doctors determined to abort the IL-2 on February 2, 2007. On August 23 there was no change to the tumors in my back or lungs but also no growth. In October 24 I got the word that the tumors and the lung nodules were shrinking. In April 14, 2008, the 40 + nodules in my lungs decrease to 2. In July 2008, the nodules in my lungs were undetectable and the ones on my back were all but one gone. Presently, I am due for another round of CT and MRI most likely for a restaging (NED)
July (2005)I was riding my bicycle to work at the Eastman Kodak Research Labs about 3 miles from home. I was wearing a knapsack to carry my things to and from the labs. I started noticing an ache on my back. I asked my wife to take a look at it. It was just a mole. So I decide to go to the dermatologist. To make the log story short, it was cancer. It took about a month and a half to get it biopsied . (Sept. 26 2005) I contacted a surgical oncologist and on October 27 2005 I had a wide incision to remove the tumor off my back. I also had a PET scan and a sentinel lymph node biopsy done and it showed no cancer except the localized one on my back. I knew from my research that I would be needing adjuvant therapy. So I started communicating with Sloan Kettering, University of Pittsburgh Cancer Center, and a couple of others including the Hillman Cancer Center and Wilmot Cancer Center at Strong in Rochester N.Y.
I am a researcher by schooling
Thanks in advance.
FREE NEJM E-TOC HOME | SUBSCRIBE | CURRENT ISSUE | PAST ISSUES | COLLECTIONS | Search Term Advanced Search
Sign in | Get NEJM's E-Mail Table of Contents — Free | Subscribe
Previous
Volume 358:2698-2703
June 19, 2008 Number 25
Treatment of Metastatic Melanoma with Autologous CD4+ T Cells against NY-ESO-1
Naomi N. Hunder, M.D., Herschel Wallen, M.D., Jianhong Cao, Ph.D., Deborah W. Hendricks, B.Sc., John Z. Reilly, B.Sc., Rebecca Rodmyre, B.Sc., Achim Jungbluth, M.D., Sacha Gnjatic, Ph.D., John A. Thompson, M.D., and Cassian Yee, M.D.
SOURCES: Cassian Yee, M.D., associate member, clinical research division, Fred Hutchinson Cancer Research Center, Seattle; Louis M. Weiner, M.D., director, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, D.C.; Vijay Trisal, M.D., assistant professor of surgical oncology, City of Hope Cancer Center, Duarte, Calif.; June 19, 2008, New England Journal of Medicine
Yessssssssss!!!!!!!!!!! I was on to Something BIG!!!!!!!!!!!!!
Response from Dr. Yee
yes
Cassian Yee MD
Associate Member
Fred Hutchinson Cancer Research Center
Seattle, WA 98109
206 667 6287 voice
206 667 7983 fax
cyee@fhcrc.org
I have That PAPER!!!!!!!!!!!!!!!!!!!!!!!!!!!
Jimmy B
Dr. Yee could you email a copy of this paper.
I had e-mail Dr. Yee on 12-9-2008 for his paper
I am a cancer patient (50 yrs. old) under the care of Dr. John Kirkwood at the Hillman Cancer Center at the University of Pittsburgh. I have gone through a wide incision, lymph nodes removal, Interferon therapy, and Dicarbazine therapy and CTLA-4 without success. I am presently washed out an IL-2 clinical trial that started in November 1, 2006. On the fourth cycle I had a heart attack and the doctors determined to abort the IL-2 on February 2, 2007. On August 23 there was no change to the tumors in my back or lungs but also no growth. In October 24 I got the word that the tumors and the lung nodules were shrinking. In April 14, 2008, the 40 + nodules in my lungs decrease to 2. In July 2008, the nodules in my lungs were undetectable and the ones on my back were all but one gone. Presently, I am due for another round of CT and MRI most likely for a restaging (NED)
July (2005)I was riding my bicycle to work at the Eastman Kodak Research Labs about 3 miles from home. I was wearing a knapsack to carry my things to and from the labs. I started noticing an ache on my back. I asked my wife to take a look at it. It was just a mole. So I decide to go to the dermatologist. To make the log story short, it was cancer. It took about a month and a half to get it biopsied . (Sept. 26 2005) I contacted a surgical oncologist and on October 27 2005 I had a wide incision to remove the tumor off my back. I also had a PET scan and a sentinel lymph node biopsy done and it showed no cancer except the localized one on my back. I knew from my research that I would be needing adjuvant therapy. So I started communicating with Sloan Kettering, University of Pittsburgh Cancer Center, and a couple of others including the Hillman Cancer Center and Wilmot Cancer Center at Strong in Rochester N.Y.
I am a researcher by schooling
Thanks in advance.
FREE NEJM E-TOC HOME | SUBSCRIBE | CURRENT ISSUE | PAST ISSUES | COLLECTIONS | Search Term Advanced Search
Sign in | Get NEJM's E-Mail Table of Contents — Free | Subscribe
Previous
Volume 358:2698-2703
June 19, 2008 Number 25
Treatment of Metastatic Melanoma with Autologous CD4+ T Cells against NY-ESO-1
Naomi N. Hunder, M.D., Herschel Wallen, M.D., Jianhong Cao, Ph.D., Deborah W. Hendricks, B.Sc., John Z. Reilly, B.Sc., Rebecca Rodmyre, B.Sc., Achim Jungbluth, M.D., Sacha Gnjatic, Ph.D., John A. Thompson, M.D., and Cassian Yee, M.D.
SOURCES: Cassian Yee, M.D., associate member, clinical research division, Fred Hutchinson Cancer Research Center, Seattle; Louis M. Weiner, M.D., director, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, D.C.; Vijay Trisal, M.D., assistant professor of surgical oncology, City of Hope Cancer Center, Duarte, Calif.; June 19, 2008, New England Journal of Medicine
Yessssssssss!!!!!!!!!!! I was on to Something BIG!!!!!!!!!!!!!
Response from Dr. Yee
yes
Cassian Yee MD
Associate Member
Fred Hutchinson Cancer Research Center
Seattle, WA 98109
206 667 6287 voice
206 667 7983 fax
cyee@fhcrc.org
I have That PAPER!!!!!!!!!!!!!!!!!!!!!!!!!!!
Jimmy B
Subscribe to:
Comments (Atom)
Greetings to One and All
This Blog is dedicated My Brother Kenny B. who passed away in the late 1970's with Cancer before the Internet.
It was he, who showed me How to live and give back. He was wise beyond his years.

Jimmy and Dee
Carepage: Jimmybreitfeller
Jimmy Breitfeller
It was he, who showed me How to live and give back. He was wise beyond his years.

Jimmy and Dee
Carepage: Jimmybreitfeller
Jimmy Breitfeller
My Profile as of 2009

- jimmy_B
- Last July (2005)I was riding my bicycle to work at the Eastman Kodak Research Labs about 3 miles from home. I was wearing a knapsack to carry my things to and from the labs. I started noticing an ache on my back. So I decide to go to the dermatologist. To make the long story short, it was cancer. I knew from my research that I would be needing adjuvant therapy. So I started communicating with Sloan Kettering, University of Pittsburgh Cancer Center, and a couple of others including the Wilmot Cancer Center at Strong. I realized that by telling my story, I might help someone else out there in a similar situation. So to all who are linked by diagnosis or by relation to someone with melanoma, I wish you well. Stay positive, read as much as you can (information helps to eliminate the fear associated with the unknown), and live for today, as no one can predict what tomorrow may bring. Jimmy B. posted 12/15/08
Disclaimer
The information contained within this Blog is not meant to replace the examination or advice of your Oncologist or Medical Team. The educational material that is covered here or Linked to, does not cover every detail of each disorder discussed.
Only your physician/Oncologist can make medical decisions and treatment plans that are appropriate for you. But, An Educated Consumer is a Smart consumer.
As Dr. Casey Culberson Said:
"The BEST melanoma patient is an ACTIVE PARTICIPANT in his or her treatment
(not a PASSIVE RECIPIENT)"
Only your physician/Oncologist can make medical decisions and treatment plans that are appropriate for you. But, An Educated Consumer is a Smart consumer.
As Dr. Casey Culberson Said:
"The BEST melanoma patient is an ACTIVE PARTICIPANT in his or her treatment
(not a PASSIVE RECIPIENT)"
Melanoma and the “Magic Bullet” (Monoclonal Antibodies)
Just to let you know I posted the first draft of the Melanoma and the “Magic Bullet” (Monoclonal Antibodies). on Melanoma Missionary In the Shared File Section. you can download it for 19.95 (Only kidding) it is Free for the taking.
It is 33 pages long and may help you in your quest for the Yellow Brick Broad. Just to let you know it is only the first draft. Revisions are sure to come. I wanted to get it to the people that need it the most, the Melanoma Patients.
Preview:
So, where does Interluekin-2 (IL-2) come into play? According to Byung-Scok et al and recent reports, IL-2 is not needed for developmental CD4+ CD25+ Treg cells in the thymus but does play an important role in the maintenance and function in the peripheral.18 Peripheral is defines as secondary system outside the bone marrow and thymus. It entails the site of antigen, immune system interaction. IL-2 is required for the peripheral generation of Tregs based Abbas’s and colleagues research.19
IL-2 prevents the spontaneous apoptosis of the CD4+ CD25+ Treg cells. It has been reported that patients with multiple advance-stage tumors have elevated levels of Tregs within the tumor microenviroment.20 Interluekin-2 is the survival factor for CD4+ CD25+ Treg cells.21 If the addition of IL-2 is on or before the maximum propagation of the CD4+ T cells, the Tregs population can increase 5-fold in a 96 hour period based on certain growth mediums.
By controlling the addition of the endogenous IL-2, one has a knob to turn and can lead to the control of the expansion of the Tregs. When you combined this control with the anti-CTLA-4 blockage, you can shift the balance of the immune response.
Now here is the catch. The maintenance and function of the CD8+ T-cells require CD4+ cells which secrete IL-2. So we don’t want to deplete the CD4+ cells, we want to control the expansion of the Tregs which are a subset of the CD4+ cells. It has been postulated by some researchers that the Anti-CTLA-4 blockage also suppresses the Treg function in a different mechanism. By using IL-2 as the rate limiting factor, we can suppress the CD4+ CD25+ Treg cell expansion by controlling the concentration and timing of the Inerluekin-2 at the tumor microenvironment.
The Interluekin-2 plays another role in this Melanoma Maze. In a study by Janas et al, Il-2 increases the expressions of the perforin and granzyme A, B and C genes in the CD8+ T-cells. This increase expression causes the CD8+ T-cells to mature into Cytoxic T Lymphocytes (CTLs). The exogenous IL-2 is required for the granzyme proteins. As stated previously, CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Once in the tumor cell, these enzymes are able to breakup (lyse) the cell and destroy it. This is the beginning of the end for the cancer cells. The tumors begin to shrink and the rest is history,
“
On the other hand, prolong therapy with Il-2 can result in causing apoptotic death of the tumor- specific CD8+ T-cells.23
Clearly in a clinical setting, timing, dose, and exposure to these drugs play a major roll in the immunotherapy, and can have dramatic effects on the outcome.
All it takes is that one magic bullet to start the immune reaction..
https://app.box.com/shared/kjgr6dkztj
Melanoma And The Magic Bullet (Monoclonal Antibodies)
It is 33 pages long and may help you in your quest for the Yellow Brick Broad. Just to let you know it is only the first draft. Revisions are sure to come. I wanted to get it to the people that need it the most, the Melanoma Patients.
Preview:
So, where does Interluekin-2 (IL-2) come into play? According to Byung-Scok et al and recent reports, IL-2 is not needed for developmental CD4+ CD25+ Treg cells in the thymus but does play an important role in the maintenance and function in the peripheral.18 Peripheral is defines as secondary system outside the bone marrow and thymus. It entails the site of antigen, immune system interaction. IL-2 is required for the peripheral generation of Tregs based Abbas’s and colleagues research.19
IL-2 prevents the spontaneous apoptosis of the CD4+ CD25+ Treg cells. It has been reported that patients with multiple advance-stage tumors have elevated levels of Tregs within the tumor microenviroment.20 Interluekin-2 is the survival factor for CD4+ CD25+ Treg cells.21 If the addition of IL-2 is on or before the maximum propagation of the CD4+ T cells, the Tregs population can increase 5-fold in a 96 hour period based on certain growth mediums.
By controlling the addition of the endogenous IL-2, one has a knob to turn and can lead to the control of the expansion of the Tregs. When you combined this control with the anti-CTLA-4 blockage, you can shift the balance of the immune response.
Now here is the catch. The maintenance and function of the CD8+ T-cells require CD4+ cells which secrete IL-2. So we don’t want to deplete the CD4+ cells, we want to control the expansion of the Tregs which are a subset of the CD4+ cells. It has been postulated by some researchers that the Anti-CTLA-4 blockage also suppresses the Treg function in a different mechanism. By using IL-2 as the rate limiting factor, we can suppress the CD4+ CD25+ Treg cell expansion by controlling the concentration and timing of the Inerluekin-2 at the tumor microenvironment.
The Interluekin-2 plays another role in this Melanoma Maze. In a study by Janas et al, Il-2 increases the expressions of the perforin and granzyme A, B and C genes in the CD8+ T-cells. This increase expression causes the CD8+ T-cells to mature into Cytoxic T Lymphocytes (CTLs). The exogenous IL-2 is required for the granzyme proteins. As stated previously, CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Once in the tumor cell, these enzymes are able to breakup (lyse) the cell and destroy it. This is the beginning of the end for the cancer cells. The tumors begin to shrink and the rest is history,
“
On the other hand, prolong therapy with Il-2 can result in causing apoptotic death of the tumor- specific CD8+ T-cells.23
Clearly in a clinical setting, timing, dose, and exposure to these drugs play a major roll in the immunotherapy, and can have dramatic effects on the outcome.
All it takes is that one magic bullet to start the immune reaction..
https://app.box.com/shared/kjgr6dkztj
Melanoma And The Magic Bullet (Monoclonal Antibodies)
Public Service Announcement
A call for Melanoma Patients by Dr. Steven A Rosenberg
"We continue to see a high rate of clinical responses in our cell transfer immunotherapy treatments for patients with metastatic melanoma", Dr. Rosenberg said.
"We are actively seeking patients for these trials and any note of that on a patient-directed web site would be appreciated."
If you would like to apply for his trials, here is the website and information.
Dr. Rosenberg's information
Dr. Rosenberg's Clinical Trials

The Melanoma Research Alliance has partnered with Bruce Springsteen, the E Street Band, and the Federici family to alleviate suffering and death from melanoma. Please view Bruce Springsteen’s public service announcement inspired by Danny Federici. Danny was the E Street Band’s organist and keyboard player. He died on April 17, 2008 at Memorial Sloan-Kettering Cancer Center in New York City after a three year battle with melanoma.
http://www.melanomaresearchalliance.org/news/PSA/
Source Fastcures blog
"We continue to see a high rate of clinical responses in our cell transfer immunotherapy treatments for patients with metastatic melanoma", Dr. Rosenberg said.
"We are actively seeking patients for these trials and any note of that on a patient-directed web site would be appreciated."
If you would like to apply for his trials, here is the website and information.
Dr. Rosenberg's information
Dr. Rosenberg's Clinical Trials

The Melanoma Research Alliance has partnered with Bruce Springsteen, the E Street Band, and the Federici family to alleviate suffering and death from melanoma. Please view Bruce Springsteen’s public service announcement inspired by Danny Federici. Danny was the E Street Band’s organist and keyboard player. He died on April 17, 2008 at Memorial Sloan-Kettering Cancer Center in New York City after a three year battle with melanoma.
http://www.melanomaresearchalliance.org/news/PSA/
Source Fastcures blog
Join the Relay for Life!!!

Dear Family and Friends,
I’ve decided to take a stand and fight back against cancer by participating in the American Cancer Society Relay For Life® event right here in my community! Please support me in this important cause by making a secure, tax-deductible donation online using the link below.
To donate on line now, click here to visit my personal page.
Jimmy B AKA Melanoma_Missionary
Relay For Life® is a life-changing event that brings together more than 3.5 million people worldwide to:
CELEBRATE the lives of those who have battled cancer. The strength of survivors inspires others to continue to fight.
REMEMBER loved ones lost to the disease. At Relay, people who have walked alongside people battling cancer can grieve and find healing.
FIGHT BACK. We Relay because we have been touched by cancer and desperately want to put an end to the disease.
Whatever you can give will help - it all adds up! I greatly appreciate your support and will keep you posted on my progress.
Keep the Fire Burning!!!

Sincerely,
Jimmy Breitfeller
Turn off Music before you "Click to Play"
Signs of Melanoma Carcinoma Skin Cancer
Signs of Melanoma Carcinoma Skin Cancer
How Skin Cancer Develops by "About.com : Dermatology"
Call for Patients with Unresectable Liver Metastases Due to Melanoma
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver.
This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Current Trial Centers
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver.
This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Current Trial Centers
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
Blog Archive
-
▼
2009
(332)
-
▼
January
(79)
- Lessons From Three Decades of Clinical Trials in M...
- State of the Science Lecture Series On Melanoma .....
- Medicare Broadens Coverage of Cancer Drugs.. Melan...
- April 14, 2008 Pfizer Anti-CTLA4 antibody trial fo...
- Follow Up of Dr. Flaherty’s Conference Call on Tar...
- Targeting Immune Stimulation: Clinical Application...
- Latest survival data from three Phase II ipilimuma...
- Visitor Map Worldwide
- Jimmy B's Update. Melanoma .. Jim Breitfeller
- Summary of the Conference Call with Dr. Flaherty o...
- Sign up and the Doors of Knowlege will Open!!!! Me...
- Hey!!!!! Look What I Found!!!!!!! Melanoma Jim Bre...
- Phase III Trial Comparing Concurrent Biochemothera...
- Call for Patients!!!!!!! NEW CLINICAL TRIAL!!!!!!!!
- Some Thing New I have found Out about Melanoma!!!!...
- Important Advance In The Treatment Of Cancer Melan...
- My search has taken me Overseas!!!! Melanoma .. J...
- Bookmark This Website!! One of the Best for Melano...
- Educational Teleconference - Cancer Care: New Adva...
- I got the "Clean and Green" from Dr. Marino .. Ji...
- I am coming up for Air!!!
- I Thought I was the Only ONE Seeing This Trend!! M...
- Can you Say Pharmacogenomics & Personalized Medici...
- Metastatic melanoma—challenges for the community o...
- You can Join too!!!!! "OncologySTAT" Melanoma jim...
- A Reply back from DR. Markovic, Svetomir N., M.D.,...
- Understanding Cancer Series: The Immune System (Na...
- Financial Help for lost income... if you got Melan...
- am Very Sadden,we lost another true Warrior Today
- The Bottom Line!!!!! Melanoma Jim Breitfeller
- Malignant melanoma in the 21st century: the emergi...
- Link Between T Cell Response To New Melanoma Antig...
- New Data On Melanoma Treatment Dicarbazine and or ...
- Impact of false-negative sentinel lymph node biops...
- Free Transport for you and your caregiver (Wife) !...
- One of our family Member asked about C kit testin...
- HLA-2a Definition Please add it to the file!!!!!!...
- DATE 12-09-2008 I contacted Dr. Cassian Yee, M.D....
- Experimental Therapy Beats Back One Patient's Mela...
- Research is Paying off!!!!!! Adoptive Cell Transfe...
- Your Reading Assignment !!!!!!
- Gene Function 'Lost' In Melanoma And Glioblastoma
- "6) Patient power." Fastercure blog jim Breitfeller
- Great News!!!!!!!!!!!! The Melanoma News Feed is C...
- Maybe I went too far???
- Behind the Sences!!!!! Jim Breitfeller
- Letter to Dr. Steven Rosenberg at the National Ca...
- Remember I said we have an Army Fighting Melanoma!...
- Scientists develop drug delivery system for brain ...
- Researchers develop novel anti-tumor vaccine
- This is for all you Women out there :Scientists fi...
- Second response from Molecular Profiling for Jim B...
- The Response to Molecular Profiling Jim Breitfelle...
- Do You Recall the paper I posted? "Adapting life s...
- Last but not Least there is Melissa!!!
- And Then There were Three!!!!!
- Larry Just posted this in the Messages
- I know I said that I would not post, I am Sorry Ji...
- I have a Confession to make!!!!! Jim Breitfeller
- Insights from molecular pathology"Another key to p...
- Insights from Molecular Pathology Melanoma.. Jim b...
- I got an Epiffany Last Night!!!!! Melanoma.. Jim B...
- This one goes out to Family Members ... MELARIS®
- While we are on the subject of Personalized Thera...
- 2008 Year in Review Continued ...Melanoma
- This one is for Doug LaVigne, The Biology Teacher
- New Approaches for Advanced Melanoma
- Most Cancer Clinical Trials Go UnpublishedFindings...
- BOY I love this INTERNET !!!!!
- Looks Who's Counting!! ME! Jim Breitfeller Progres...
- 2008 Doctors sequence entire genome of a cancer pa...
- FasterCures’ Ten to Watch in 2009: Bigger Bang for...
- There is Nothing like a ROSE!!!!!!
- Adjuvant Therapy in Melanoma: New Combination Cyto...
- First targeted therapy for melanoma brings Hope!!
- Scientists identify interacting proteins key to me...
- Year of the Cure??????? Melanoma
- 2008's 1 of 12 Major Cancer Advances
- Quantifying cytokine levels to differentiate betwe...
-
▼
January
(79)
Call For Melanoma Patients!!!!
Call For Melanoma Patients!!!!
Dr. Rosenberg Has a New Clinical Trial.
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Dr. Rosenberg's Clinical Trials
(For a copy of the research paper.. see My Shared files)
Dr. Rosenberg Has a New Clinical Trial.
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Dr. Rosenberg's Clinical Trials
(For a copy of the research paper.. see My Shared files)
The news headlines shown above for Melanoma / Skin Cancer are provided courtesy of Medical News Today.
