This is Jim Breitfeller's journey into the Maze of Melanoma. Jim Breitfeller has gathered medical information for the patient and the caregiver. As Lance Armstrong would say "Lets stand Up to Cancer" Jim's Battle with the Beast July 2005 to present.
Wednesday, May 15, 2013
Thursday, January 10, 2013
Pearls of Wisdom: Melanoma ..Jim Breitfeller
Pearls of Wisdom: Melanoma
Reviewed by Douglas Johnson, MD and Jeffrey Sosman, MD
This entry was posted on Tuesday, January 8, 2013 at 6:33 pm Management of Advanced Disease
Metastatic melanoma historically has had a very poor prognosis with a median survival of 6-9 months. However, recent advances in molecularly targeted therapy as well as immune based therapies have provided several effective treatment options for oncologists and their patients. In 2012 there are several important issues to consider in the management of patients with newly diagnosed advanced melanoma.
Advanced Stage III/Stage IV resectable disease
• In patients with advanced stage III disease or oligometastatic disease, consultation with an experienced surgical oncologist is warranted, as patients can experience long term survival with an aggressive surgical approach. Despite the advances in systemic therapy, surgical resection is still our preferred option in most of these clinical situations. One series of 64 patients showed a >30% four year survival in patients who had completely resected metastatic disease.1
•Another option we have offered is a short course of systemic therapy for 6-12 months prior to surgery with the intent to improve the chance that rapid recurrence of disease does not occur and to define the tumor’s responsiveness to the treatment.
•Patients should be considered for clinical trials in the adjuvant setting. Studies are ongoing to assess whether ipilimumab or vemurafenib is effective in preventing recurrences in high risk patients. For stage IV (M1) disease after complete surgical treatment there is no standard of care adjuvant treatment.
•Interferon, the current standard of care for fully resected stage IIb-III disease, is also an option in the adjuvant setting.
Molecular Testing
•All patients with metastatic disease should undergo testing for BRAF V600 mutations given the availability of targeted therapy for this mutation. This should include testing for the alternate V600 mutations that make up about 20% of BRAF mutations at this codon and appear sensitive to the BRAF inhibitors such as vemurafenib and dabrafenib.2 Patients with advanced local or regional disease can also be considered for testing since there are a number of clinical trials either recently or soon to be activated with BRAF inhibitor based therapy in BRAF V600 mutated melanoma at stage IIC-IIIC. Additionally, results will be available on progression so that therapy can be rapidly initiated if needed.
•Evaluation of several KIT mutations should also be considered in at least specific clinically defined subsets of patients. Though KIT mutations are present in only ~2% of all melanomas, acral (feet and hands) and mucosal melanomas harbor KIT mutations in 15-20% of cases. KIT mutations are sensitive to imatinib in about 25% of the cases based on limited numbers of patients.3
•NRAS mutations are also present in 15-20% of patients and though no targeted therapies are yet established as standard for this subset, there are a number of studies underway specifically targeting this mutation. Furthermore, the NRAS mutation may confer a poor prognosis to the melanoma.4
Standard therapies for metastatic disease
Vemurafenib
Vemurafenib is a BRAF inhibitor approved for patients with metastatic melanoma with BRAF V600 mutations (40-50%). In phase III trial data, objective tumor shrinkage was seen in well over 50% of patients, and clinical benefit seen in nearly all patients during the initial few weeks of administration. Median progression-free survival was 6.9 months (compared to 1.6 months with dacarbazine).5 Some important points regarding vemurafenib therapy:
•Responses are often rapid and dramatic. If patients with mutated BRAF V600 melanoma are highly symptomatic, vemurafenib would be our first line therapy in nearly all such patients.
•Patients will inevitably progress or relapse, often within the first year. Strategies to prevent or delay resistance are being pursued. In fact, recently the addition of a MEK inhibitor, trametinib, demonstrated an ability to improve the frequency of objective responses and the duration of progression-free survival in those melanoma patients receiving dabrafenib (BRAF inhibitor).
•Vemurafenib has clinical activity in patients with brain metastases in small series. Prospective phase II trials are pending completion. Trials with dabrafenib have also demonstrated frequent clinical benefits in such patients.6
•Secondary squamous cell carcinomas are common in BRAF inhibitor trials (10-25%).7 Suspicious lesions should be biopsied. Additionally, secondary melanomas and CMML have been reported in the presence of vemurafenib; almost certainly the drug has played a role to accelerate the appearance and growth of these tumors.
Ipilimumab (Yervoy)
Ipilimumab is an antibody to CTLA-4 and functions as an immune checkpoint inhibitor. This “removes the brakes” on the immune system which decreases immune tolerance to the tumor but may also cause autoimmune toxicities. In phase III trials, overall survival was increased compared to a vaccine in previously treated patients (10 mos vs. 6.4 mos), and in combination with dacarbazine compared with dacarbazine alone in the first line setting (11.2 vs. 9.1 months).8,9 Some important points to consider:
•Tumor burden on exam or on imaging may transiently worsen even in patients destined to respond. We do not repeat imaging until after 12 weeks of therapy unless the patient develops concerning new or progressive symptoms.
•Since even responding patients may have tumor growth or slow shrinkage, we are hesitant to use this therapy in highly symptomatic patients, especially if there are reasonable alternatives.
•Autoimmune side effects may be severe. Colitis, hepatitis, dermatitis, neuropathy, and endocrinopathies are the most common toxicities.
•Patients should be monitored closely for colitis. Concerning symptoms (diarrhea, melena/hematochezia, abdominal pain) require rapid evaluation. Though Imodium is appropriate for mild diarrhea (grade I), grade II toxicities or worse require prompt initiation of steroids. Colonoscopy should be performed to confirm the diagnosis in patients with severe colitis. Bowel perforation and death have been reported in extreme cases almost always when initial therapy is delayed.
•Headaches, with or without vision changes and fatigue may represent hypophysitis. Evaluation of TSH, cortisol, and testosterone, and brain MRI can aid in this diagnosis.
•We monitor TSH, cortisol, and liver enzymes at every visit.
•Other rare immune mediated side effects such as Guillan Barre syndrome, myasthenia gravis, sarcoidosis, and hemophilia have also been described.
Interleukin-2 (IL-2)
IL-2 has been used for many years in treating metastatic melanoma. It is associated with 6% durable remission rate (cures) and 15-20% overall response rate.10 Its use is limited to centers familiar with the associated severe side effects. Some points to consider when treating or referring patients:
•Therapy is limited to healthy patients usually under 70 years of age with adequate cardiac, pulmonary, renal, hepatic, and hematopoietic organ function and well controlled brain metastases. Nearly all patients experience treatment associated hypotension, multi-organ dysfunction, and vascular leak syndrome
•Though newer agents exist, IL-2 has the most long term data showing durable remissions in the minority of patients. We still consider this therapy in interested, eligible patients.
•Advantages of using IL-2 compared to ipilimumab include longer duration of use and more rapid assessments of benefit (8 weeks vs. 12+ weeks). Advantages to ipilimumab include outpatient use, generally less severe side effects, and possibly higher rates of durable remissions.
Cytotoxic chemotherapy
•Dacarbazine, temazolamide, and carboplatin/paclitaxel occasionally benefit patients with metastatic disease.
•No consistent survival benefit has been demonstrated with cytotoxic chemotherapy. Response rates are typically in the 5-10% range.
•We use chemotherapy only in patients who are not eligible for other therapies.
Which therapy should be chosen as first line therapy in patients with BRAF V600E/K mutations?
•This decision should be individualized. No head to head comparisons have been done with immunotherapy in the form of ipilimumab or interleukin-2 therapy. BRAF inhibitors are associated with high response rates but inevitable progression, while immune therapies have low objective response rates but durable remissions in a minority of patients.
•In patients with rapidly progressive or highly symptomatic disease, we typically use vemurafenib. In patients with asymptomatic disease, we favor immune based therapies for interested patients. An intergroup randomized cross over trial will address this issue, where half the patients start with vemurafenib and the other half on ipilimumab.
Promising Therapies
Therapy for metastatic melanoma is rapidly evolving. Many clinical trials are ongoing, and patients should be encouraged to participate in clinical trials for first line therapy or at progression if treated with standard therapy options. Some therapies that are likely to be approved are listed below.
•BRAF/MEK inhibitor combination therapies: Dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) have both shown benefit individually over dacarbazine in phase III trials in patients with BRAF mutant metastatic melanoma. However, interest lies especially in combination therapy to delay resistance and disease progression. In a randomized phase II trial, combination therapy was associated with an improved objective response rate (54% vs 76%) and progression free survival compared to dabrafenib alone (9.4mo vs. 5.8mo).11 Additionally, the incidence of squamous cell carcinomas was dramatically decreased with combination therapy. Trials are ongoing comparing this combination with vemurafenib in untreated patients with BRAF mutations. An additional trial is evaluating vemurafenib in combination with GDC-0973, a MEK inhibitor.
•Anti-PD-1 therapy:
Several newer checkpoint inhibitors block the interaction between PD-1 (expressed on T-cells) and PD-L1 (expressed on tumor) which activates the immune system, hopefully in a tumor specific manner. A phase I trial with nivolumab (anti-PD1 BMS) data shows a 31% response rate with fewer autoimmune side effects than ipilimumab.12 Pneumonitis was described in several patients. Trials are ongoing in patients who have progressed on ipilimumab. Early reports of another anti-PD1, MK-3475 also show a remarkable response rate in patients either following Ipilimumab or de novo in the 40-50% response range.
•Anti-PD-1 + Yervoy therapy:
By using both Checkpoint inhibitors, you and block two pathways that the melanoma can't use for immunosuppression. This allows the activated T-cells to go unchecked and keeps the immune response switch on. This clinical trial is ongoing at Sloan Kettering, and Yale. Rumor has it that they are seeing high response rates.
References
1.
Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: Results of Southwest Oncology Group Clinical Trial S9430. Cancer 2011.
2.
Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PloS one 2012;7:e35309.
3.
Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA : the journal of the American Medical Association 2011;305:2327-34.
4.
Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment cell & melanoma research 2011;24:666-72.
5.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine 2011;364:2507-16. “It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.”
~Charles Darwin~
Take Care,
Jimmy B

Reviewed by Douglas Johnson, MD and Jeffrey Sosman, MD
This entry was posted on Tuesday, January 8, 2013 at 6:33 pm Management of Advanced Disease
Metastatic melanoma historically has had a very poor prognosis with a median survival of 6-9 months. However, recent advances in molecularly targeted therapy as well as immune based therapies have provided several effective treatment options for oncologists and their patients. In 2012 there are several important issues to consider in the management of patients with newly diagnosed advanced melanoma.
Advanced Stage III/Stage IV resectable disease
• In patients with advanced stage III disease or oligometastatic disease, consultation with an experienced surgical oncologist is warranted, as patients can experience long term survival with an aggressive surgical approach. Despite the advances in systemic therapy, surgical resection is still our preferred option in most of these clinical situations. One series of 64 patients showed a >30% four year survival in patients who had completely resected metastatic disease.1
•Another option we have offered is a short course of systemic therapy for 6-12 months prior to surgery with the intent to improve the chance that rapid recurrence of disease does not occur and to define the tumor’s responsiveness to the treatment.
•Patients should be considered for clinical trials in the adjuvant setting. Studies are ongoing to assess whether ipilimumab or vemurafenib is effective in preventing recurrences in high risk patients. For stage IV (M1) disease after complete surgical treatment there is no standard of care adjuvant treatment.
•Interferon, the current standard of care for fully resected stage IIb-III disease, is also an option in the adjuvant setting.
Molecular Testing
•All patients with metastatic disease should undergo testing for BRAF V600 mutations given the availability of targeted therapy for this mutation. This should include testing for the alternate V600 mutations that make up about 20% of BRAF mutations at this codon and appear sensitive to the BRAF inhibitors such as vemurafenib and dabrafenib.2 Patients with advanced local or regional disease can also be considered for testing since there are a number of clinical trials either recently or soon to be activated with BRAF inhibitor based therapy in BRAF V600 mutated melanoma at stage IIC-IIIC. Additionally, results will be available on progression so that therapy can be rapidly initiated if needed.
•Evaluation of several KIT mutations should also be considered in at least specific clinically defined subsets of patients. Though KIT mutations are present in only ~2% of all melanomas, acral (feet and hands) and mucosal melanomas harbor KIT mutations in 15-20% of cases. KIT mutations are sensitive to imatinib in about 25% of the cases based on limited numbers of patients.3
•NRAS mutations are also present in 15-20% of patients and though no targeted therapies are yet established as standard for this subset, there are a number of studies underway specifically targeting this mutation. Furthermore, the NRAS mutation may confer a poor prognosis to the melanoma.4
Standard therapies for metastatic disease
Vemurafenib
Vemurafenib is a BRAF inhibitor approved for patients with metastatic melanoma with BRAF V600 mutations (40-50%). In phase III trial data, objective tumor shrinkage was seen in well over 50% of patients, and clinical benefit seen in nearly all patients during the initial few weeks of administration. Median progression-free survival was 6.9 months (compared to 1.6 months with dacarbazine).5 Some important points regarding vemurafenib therapy:
•Responses are often rapid and dramatic. If patients with mutated BRAF V600 melanoma are highly symptomatic, vemurafenib would be our first line therapy in nearly all such patients.
•Patients will inevitably progress or relapse, often within the first year. Strategies to prevent or delay resistance are being pursued. In fact, recently the addition of a MEK inhibitor, trametinib, demonstrated an ability to improve the frequency of objective responses and the duration of progression-free survival in those melanoma patients receiving dabrafenib (BRAF inhibitor).
•Vemurafenib has clinical activity in patients with brain metastases in small series. Prospective phase II trials are pending completion. Trials with dabrafenib have also demonstrated frequent clinical benefits in such patients.6
•Secondary squamous cell carcinomas are common in BRAF inhibitor trials (10-25%).7 Suspicious lesions should be biopsied. Additionally, secondary melanomas and CMML have been reported in the presence of vemurafenib; almost certainly the drug has played a role to accelerate the appearance and growth of these tumors.
Ipilimumab (Yervoy)
Ipilimumab is an antibody to CTLA-4 and functions as an immune checkpoint inhibitor. This “removes the brakes” on the immune system which decreases immune tolerance to the tumor but may also cause autoimmune toxicities. In phase III trials, overall survival was increased compared to a vaccine in previously treated patients (10 mos vs. 6.4 mos), and in combination with dacarbazine compared with dacarbazine alone in the first line setting (11.2 vs. 9.1 months).8,9 Some important points to consider:
•Tumor burden on exam or on imaging may transiently worsen even in patients destined to respond. We do not repeat imaging until after 12 weeks of therapy unless the patient develops concerning new or progressive symptoms.
•Since even responding patients may have tumor growth or slow shrinkage, we are hesitant to use this therapy in highly symptomatic patients, especially if there are reasonable alternatives.
•Autoimmune side effects may be severe. Colitis, hepatitis, dermatitis, neuropathy, and endocrinopathies are the most common toxicities.
•Patients should be monitored closely for colitis. Concerning symptoms (diarrhea, melena/hematochezia, abdominal pain) require rapid evaluation. Though Imodium is appropriate for mild diarrhea (grade I), grade II toxicities or worse require prompt initiation of steroids. Colonoscopy should be performed to confirm the diagnosis in patients with severe colitis. Bowel perforation and death have been reported in extreme cases almost always when initial therapy is delayed.
•Headaches, with or without vision changes and fatigue may represent hypophysitis. Evaluation of TSH, cortisol, and testosterone, and brain MRI can aid in this diagnosis.
•We monitor TSH, cortisol, and liver enzymes at every visit.
•Other rare immune mediated side effects such as Guillan Barre syndrome, myasthenia gravis, sarcoidosis, and hemophilia have also been described.
Interleukin-2 (IL-2)
IL-2 has been used for many years in treating metastatic melanoma. It is associated with 6% durable remission rate (cures) and 15-20% overall response rate.10 Its use is limited to centers familiar with the associated severe side effects. Some points to consider when treating or referring patients:
•Therapy is limited to healthy patients usually under 70 years of age with adequate cardiac, pulmonary, renal, hepatic, and hematopoietic organ function and well controlled brain metastases. Nearly all patients experience treatment associated hypotension, multi-organ dysfunction, and vascular leak syndrome
•Though newer agents exist, IL-2 has the most long term data showing durable remissions in the minority of patients. We still consider this therapy in interested, eligible patients.
•Advantages of using IL-2 compared to ipilimumab include longer duration of use and more rapid assessments of benefit (8 weeks vs. 12+ weeks). Advantages to ipilimumab include outpatient use, generally less severe side effects, and possibly higher rates of durable remissions.
Cytotoxic chemotherapy
•Dacarbazine, temazolamide, and carboplatin/paclitaxel occasionally benefit patients with metastatic disease.
•No consistent survival benefit has been demonstrated with cytotoxic chemotherapy. Response rates are typically in the 5-10% range.
•We use chemotherapy only in patients who are not eligible for other therapies.
Which therapy should be chosen as first line therapy in patients with BRAF V600E/K mutations?
•This decision should be individualized. No head to head comparisons have been done with immunotherapy in the form of ipilimumab or interleukin-2 therapy. BRAF inhibitors are associated with high response rates but inevitable progression, while immune therapies have low objective response rates but durable remissions in a minority of patients.
•In patients with rapidly progressive or highly symptomatic disease, we typically use vemurafenib. In patients with asymptomatic disease, we favor immune based therapies for interested patients. An intergroup randomized cross over trial will address this issue, where half the patients start with vemurafenib and the other half on ipilimumab.
Promising Therapies
Therapy for metastatic melanoma is rapidly evolving. Many clinical trials are ongoing, and patients should be encouraged to participate in clinical trials for first line therapy or at progression if treated with standard therapy options. Some therapies that are likely to be approved are listed below.
•BRAF/MEK inhibitor combination therapies: Dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) have both shown benefit individually over dacarbazine in phase III trials in patients with BRAF mutant metastatic melanoma. However, interest lies especially in combination therapy to delay resistance and disease progression. In a randomized phase II trial, combination therapy was associated with an improved objective response rate (54% vs 76%) and progression free survival compared to dabrafenib alone (9.4mo vs. 5.8mo).11 Additionally, the incidence of squamous cell carcinomas was dramatically decreased with combination therapy. Trials are ongoing comparing this combination with vemurafenib in untreated patients with BRAF mutations. An additional trial is evaluating vemurafenib in combination with GDC-0973, a MEK inhibitor.
•Anti-PD-1 therapy:
Several newer checkpoint inhibitors block the interaction between PD-1 (expressed on T-cells) and PD-L1 (expressed on tumor) which activates the immune system, hopefully in a tumor specific manner. A phase I trial with nivolumab (anti-PD1 BMS) data shows a 31% response rate with fewer autoimmune side effects than ipilimumab.12 Pneumonitis was described in several patients. Trials are ongoing in patients who have progressed on ipilimumab. Early reports of another anti-PD1, MK-3475 also show a remarkable response rate in patients either following Ipilimumab or de novo in the 40-50% response range.
•Anti-PD-1 + Yervoy therapy:
By using both Checkpoint inhibitors, you and block two pathways that the melanoma can't use for immunosuppression. This allows the activated T-cells to go unchecked and keeps the immune response switch on. This clinical trial is ongoing at Sloan Kettering, and Yale. Rumor has it that they are seeing high response rates.
References
1.
Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: Results of Southwest Oncology Group Clinical Trial S9430. Cancer 2011.
2.
Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PloS one 2012;7:e35309.
3.
Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA : the journal of the American Medical Association 2011;305:2327-34.
4.
Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment cell & melanoma research 2011;24:666-72.
5.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine 2011;364:2507-16. “It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.”
~Charles Darwin~
Take Care,
Jimmy B

Monday, January 7, 2013
ASCO 2013.. The comming out party for Combination Therapy!!! Yervoy + Anti-PD-1 ..Melanoma. Jim Breitfeller
 ASCO 2012 was PD-1’s debutant ball, but we may have found a partner (Yervoy) at this Year’s 2013 Ball and may be only a few years away from its coronation (FDA’s approval) as a stand alone or combinatorial therapy. I believe that ASCO 2013 will be the coming out party for combination therapy of Anti-PD-1 + Yervoy.
ASCO 2012 was PD-1’s debutant ball, but we may have found a partner (Yervoy) at this Year’s 2013 Ball and may be only a few years away from its coronation (FDA’s approval) as a stand alone or combinatorial therapy. I believe that ASCO 2013 will be the coming out party for combination therapy of Anti-PD-1 + Yervoy.br />
Using this combination blocks two checkpoint pathways on the T-Cells leaving it activated to proliferate and destroy the cancer.
If you add Yervoy & Anti-PD-1 to the therapy you have a better chance to activate the CD4 and CD8 T-cells
“It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.”
~Charles Darwin~
Take Care,
Jimmy B

Labels:
anti-CTLA-4 blockade,
anti-PD-1,
asco,
BMS,
BMY,
Bristol-Meyer Squibb,
combinatorial therapy,
Yervoy
Subscribe to:
Comments (Atom)
Greetings to One and All
This Blog is dedicated My Brother Kenny B. who passed away in the late 1970's with Cancer before the Internet.
It was he, who showed me How to live and give back. He was wise beyond his years.

Jimmy and Dee
Carepage: Jimmybreitfeller
Jimmy Breitfeller
It was he, who showed me How to live and give back. He was wise beyond his years.

Jimmy and Dee
Carepage: Jimmybreitfeller
Jimmy Breitfeller
My Profile as of 2009

- jimmy_B
- Last July (2005)I was riding my bicycle to work at the Eastman Kodak Research Labs about 3 miles from home. I was wearing a knapsack to carry my things to and from the labs. I started noticing an ache on my back. So I decide to go to the dermatologist. To make the long story short, it was cancer. I knew from my research that I would be needing adjuvant therapy. So I started communicating with Sloan Kettering, University of Pittsburgh Cancer Center, and a couple of others including the Wilmot Cancer Center at Strong. I realized that by telling my story, I might help someone else out there in a similar situation. So to all who are linked by diagnosis or by relation to someone with melanoma, I wish you well. Stay positive, read as much as you can (information helps to eliminate the fear associated with the unknown), and live for today, as no one can predict what tomorrow may bring. Jimmy B. posted 12/15/08
Disclaimer
The information contained within this Blog is not meant to replace the examination or advice of your Oncologist or Medical Team. The educational material that is covered here or Linked to, does not cover every detail of each disorder discussed.
Only your physician/Oncologist can make medical decisions and treatment plans that are appropriate for you. But, An Educated Consumer is a Smart consumer.
As Dr. Casey Culberson Said:
"The BEST melanoma patient is an ACTIVE PARTICIPANT in his or her treatment
(not a PASSIVE RECIPIENT)"
Only your physician/Oncologist can make medical decisions and treatment plans that are appropriate for you. But, An Educated Consumer is a Smart consumer.
As Dr. Casey Culberson Said:
"The BEST melanoma patient is an ACTIVE PARTICIPANT in his or her treatment
(not a PASSIVE RECIPIENT)"
Melanoma and the “Magic Bullet” (Monoclonal Antibodies)
Just to let you know I posted the first draft of the Melanoma and the “Magic Bullet” (Monoclonal Antibodies). on Melanoma Missionary In the Shared File Section. you can download it for 19.95 (Only kidding) it is Free for the taking.
It is 33 pages long and may help you in your quest for the Yellow Brick Broad. Just to let you know it is only the first draft. Revisions are sure to come. I wanted to get it to the people that need it the most, the Melanoma Patients.
Preview:
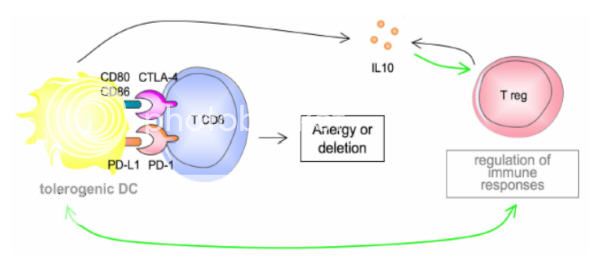
So, where does Interluekin-2 (IL-2) come into play? According to Byung-Scok et al and recent reports, IL-2 is not needed for developmental CD4+ CD25+ Treg cells in the thymus but does play an important role in the maintenance and function in the peripheral.18 Peripheral is defines as secondary system outside the bone marrow and thymus. It entails the site of antigen, immune system interaction. IL-2 is required for the peripheral generation of Tregs based Abbas’s and colleagues research.19
IL-2 prevents the spontaneous apoptosis of the CD4+ CD25+ Treg cells. It has been reported that patients with multiple advance-stage tumors have elevated levels of Tregs within the tumor microenviroment.20 Interluekin-2 is the survival factor for CD4+ CD25+ Treg cells.21 If the addition of IL-2 is on or before the maximum propagation of the CD4+ T cells, the Tregs population can increase 5-fold in a 96 hour period based on certain growth mediums.
By controlling the addition of the endogenous IL-2, one has a knob to turn and can lead to the control of the expansion of the Tregs. When you combined this control with the anti-CTLA-4 blockage, you can shift the balance of the immune response.
Now here is the catch. The maintenance and function of the CD8+ T-cells require CD4+ cells which secrete IL-2. So we don’t want to deplete the CD4+ cells, we want to control the expansion of the Tregs which are a subset of the CD4+ cells. It has been postulated by some researchers that the Anti-CTLA-4 blockage also suppresses the Treg function in a different mechanism. By using IL-2 as the rate limiting factor, we can suppress the CD4+ CD25+ Treg cell expansion by controlling the concentration and timing of the Inerluekin-2 at the tumor microenvironment.
The Interluekin-2 plays another role in this Melanoma Maze. In a study by Janas et al, Il-2 increases the expressions of the perforin and granzyme A, B and C genes in the CD8+ T-cells. This increase expression causes the CD8+ T-cells to mature into Cytoxic T Lymphocytes (CTLs). The exogenous IL-2 is required for the granzyme proteins. As stated previously, CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Once in the tumor cell, these enzymes are able to breakup (lyse) the cell and destroy it. This is the beginning of the end for the cancer cells. The tumors begin to shrink and the rest is history,
“
On the other hand, prolong therapy with Il-2 can result in causing apoptotic death of the tumor- specific CD8+ T-cells.23
Clearly in a clinical setting, timing, dose, and exposure to these drugs play a major roll in the immunotherapy, and can have dramatic effects on the outcome.
All it takes is that one magic bullet to start the immune reaction..
https://app.box.com/shared/kjgr6dkztj
Melanoma And The Magic Bullet (Monoclonal Antibodies)
It is 33 pages long and may help you in your quest for the Yellow Brick Broad. Just to let you know it is only the first draft. Revisions are sure to come. I wanted to get it to the people that need it the most, the Melanoma Patients.
Preview:
So, where does Interluekin-2 (IL-2) come into play? According to Byung-Scok et al and recent reports, IL-2 is not needed for developmental CD4+ CD25+ Treg cells in the thymus but does play an important role in the maintenance and function in the peripheral.18 Peripheral is defines as secondary system outside the bone marrow and thymus. It entails the site of antigen, immune system interaction. IL-2 is required for the peripheral generation of Tregs based Abbas’s and colleagues research.19
IL-2 prevents the spontaneous apoptosis of the CD4+ CD25+ Treg cells. It has been reported that patients with multiple advance-stage tumors have elevated levels of Tregs within the tumor microenviroment.20 Interluekin-2 is the survival factor for CD4+ CD25+ Treg cells.21 If the addition of IL-2 is on or before the maximum propagation of the CD4+ T cells, the Tregs population can increase 5-fold in a 96 hour period based on certain growth mediums.
By controlling the addition of the endogenous IL-2, one has a knob to turn and can lead to the control of the expansion of the Tregs. When you combined this control with the anti-CTLA-4 blockage, you can shift the balance of the immune response.
Now here is the catch. The maintenance and function of the CD8+ T-cells require CD4+ cells which secrete IL-2. So we don’t want to deplete the CD4+ cells, we want to control the expansion of the Tregs which are a subset of the CD4+ cells. It has been postulated by some researchers that the Anti-CTLA-4 blockage also suppresses the Treg function in a different mechanism. By using IL-2 as the rate limiting factor, we can suppress the CD4+ CD25+ Treg cell expansion by controlling the concentration and timing of the Inerluekin-2 at the tumor microenvironment.
The Interluekin-2 plays another role in this Melanoma Maze. In a study by Janas et al, Il-2 increases the expressions of the perforin and granzyme A, B and C genes in the CD8+ T-cells. This increase expression causes the CD8+ T-cells to mature into Cytoxic T Lymphocytes (CTLs). The exogenous IL-2 is required for the granzyme proteins. As stated previously, CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Once in the tumor cell, these enzymes are able to breakup (lyse) the cell and destroy it. This is the beginning of the end for the cancer cells. The tumors begin to shrink and the rest is history,
“
On the other hand, prolong therapy with Il-2 can result in causing apoptotic death of the tumor- specific CD8+ T-cells.23
Clearly in a clinical setting, timing, dose, and exposure to these drugs play a major roll in the immunotherapy, and can have dramatic effects on the outcome.
All it takes is that one magic bullet to start the immune reaction..
https://app.box.com/shared/kjgr6dkztj
Melanoma And The Magic Bullet (Monoclonal Antibodies)
Public Service Announcement
A call for Melanoma Patients by Dr. Steven A Rosenberg
"We continue to see a high rate of clinical responses in our cell transfer immunotherapy treatments for patients with metastatic melanoma", Dr. Rosenberg said.
"We are actively seeking patients for these trials and any note of that on a patient-directed web site would be appreciated."
If you would like to apply for his trials, here is the website and information.
Dr. Rosenberg's information
Dr. Rosenberg's Clinical Trials

The Melanoma Research Alliance has partnered with Bruce Springsteen, the E Street Band, and the Federici family to alleviate suffering and death from melanoma. Please view Bruce Springsteen’s public service announcement inspired by Danny Federici. Danny was the E Street Band’s organist and keyboard player. He died on April 17, 2008 at Memorial Sloan-Kettering Cancer Center in New York City after a three year battle with melanoma.
http://www.melanomaresearchalliance.org/news/PSA/
Source Fastcures blog
"We continue to see a high rate of clinical responses in our cell transfer immunotherapy treatments for patients with metastatic melanoma", Dr. Rosenberg said.
"We are actively seeking patients for these trials and any note of that on a patient-directed web site would be appreciated."
If you would like to apply for his trials, here is the website and information.
Dr. Rosenberg's information
Dr. Rosenberg's Clinical Trials

The Melanoma Research Alliance has partnered with Bruce Springsteen, the E Street Band, and the Federici family to alleviate suffering and death from melanoma. Please view Bruce Springsteen’s public service announcement inspired by Danny Federici. Danny was the E Street Band’s organist and keyboard player. He died on April 17, 2008 at Memorial Sloan-Kettering Cancer Center in New York City after a three year battle with melanoma.
http://www.melanomaresearchalliance.org/news/PSA/
Source Fastcures blog
Join the Relay for Life!!!

Dear Family and Friends,
I’ve decided to take a stand and fight back against cancer by participating in the American Cancer Society Relay For Life® event right here in my community! Please support me in this important cause by making a secure, tax-deductible donation online using the link below.
To donate on line now, click here to visit my personal page.
Jimmy B AKA Melanoma_Missionary
Relay For Life® is a life-changing event that brings together more than 3.5 million people worldwide to:
CELEBRATE the lives of those who have battled cancer. The strength of survivors inspires others to continue to fight.
REMEMBER loved ones lost to the disease. At Relay, people who have walked alongside people battling cancer can grieve and find healing.
FIGHT BACK. We Relay because we have been touched by cancer and desperately want to put an end to the disease.
Whatever you can give will help - it all adds up! I greatly appreciate your support and will keep you posted on my progress.
Keep the Fire Burning!!!

Sincerely,
Jimmy Breitfeller
Turn off Music before you "Click to Play"
Signs of Melanoma Carcinoma Skin Cancer
Signs of Melanoma Carcinoma Skin Cancer
How Skin Cancer Develops by "About.com : Dermatology"
Call for Patients with Unresectable Liver Metastases Due to Melanoma
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver.
This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Current Trial Centers
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver.
This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Current Trial Centers
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
Call For Melanoma Patients!!!!
Call For Melanoma Patients!!!!
Dr. Rosenberg Has a New Clinical Trial.
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Dr. Rosenberg's Clinical Trials
(For a copy of the research paper.. see My Shared files)
Dr. Rosenberg Has a New Clinical Trial.
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Dr. Rosenberg's Clinical Trials
(For a copy of the research paper.. see My Shared files)
The news headlines shown above for Melanoma / Skin Cancer are provided courtesy of Medical News Today.