This short paper is dedicated to all the Melanoma Patients that didnot get a complete response with IL-2 Therapy.
“Administration of (IL-2) Interluekin-2 during the initial phase of the response, clonal expansion and development of the effector function had no effect on the number of (CTL) Cytotoxic T Lymphocytes generated or control of tumor growth. In contrast, a short two day times course of low dose IL-2 at the peak of clonal expansion or at later times resulted in prolonged and expanded responses by the OT-1 CTL, with concomitant decrease in tumor load (Shrinkage) and extension of Survival.”1
An antigen (Ag) recognition event is not predetermined. T-cells that are stimulated by self Ag generally fails to proliferate effectively and undergo induced Apoptosis (programmed cell death) or develop clonal anergy (lack of energy leading to inactivity).
T-cells that are activated with non-self Antigen (Tumor specific antigen) undergo robust clonal expansion that leads to a large generation of effector T-cells, clearance of the foreign antigen-tumors, as well as development of memory cells.
A new cell growth model: IL-2 T-cell system has been postulated by K A Smith and colleagues. In their model, they show that three factors are critical for T-cell cycle progression.
1. Interleukin-2 concentration
2. Interleukin-2 receptor density on the T-cells
3. The duration of the interleukin-2 receptor interaction
Since we only have somewhat of control over Interleukin-2 concentration, the rest of the paper will focus on IL-2 concentration.
By limiting the exogenous IL-2 at the initial response phase, the T-cells in particular, the CD4 + and the CD8 + T-cells have to compete for the IL-2 that is secreted from the cells after activation. It is generally accepted that the T-cells must secrete IL-2 to aid in the expansion and survival of the T-cells. With this clonal expansion of the CD4 + T-cells also generates more subsets including the T-Regulatory foxp3 (CD4+ CD25+ FoxP3).
Tregs cells are notorious for suppressing an immune response. The Tregs cells make up
only 2-5 percent of the overall population of the CD4 + T-cells.
It has been shown that a 4-6 hour window of time was enough time to program the CD4 + T-cells to proliferate even after the separation of the antigen bearing presentation cell (APC). This activation time reflects the time needed to up-regulate the high affinity IL-2 receptor and secrete IL-2, thus facilitation of the IL-2 dependent expanding CD4 + T-cells.
It has been indicated Mueller et al that sustained (TCR) T-cell receptor and CD28 signaling is necessary to maintain an optimal rate of cell division in the presents of IL-2.
Once the antigen is cleared (used up) the CD4 + T-cells stop proliferating. So if you don’t have enough tumor specific antigens that are shed from the tumor, the expansion halts.
In a paper entitled “IL-2 Regulates Expansion of the CD4 + T-cell population by affecting Cell death, Ganusov and colleagues reported that the Il-2 has the most profound effect on the death rate of dividing CD4 + T-cells, specifically that the death rate increases with division number. Division number is the number of times the cells divide.
At the lowest IL-2 concentration, the CD4 + T-cells have the highest death rate over the divisions. They reported at the first cell division they encountered 20% of the cells dying. At the sixth division, the death rate increased to 90 %.

With the T-cells competing for IL-2, the consumption of the Interluekin-2 by the dividing CD4 + T-cells may cause the overall concentration in ones body to decrease causing a premature death to the T-cells. Without CD4 + helper cells, the CD8 + T-cells won’t get cross primed and activated. These CD4+ cells, called helper T cells, bind to antigen presented by B cells. The result is the development of clones of plasma cells secreting antibodies against the antigenic material.
As you can see it is a delicate balancing act. It is like the three bears, you need it just right. To little IL-2 will induce cell death and to much can cause a change in the feedback response loop causing the Tregs to undergo robust clonal expansion shutting down any chance of an immune response.
By increasing IL-2 concentration at the maximum propagation of the CD8+ T-cells, you actually extend the survival and the effector function of the CD8+ T-cells to mature into
Cytoxic T. Lymphocytes (CTLs).
The Interluekin-2 plays another role in this Melanoma Maze. In a study by Janas et al, IL-2 increases the expressions of the perforin and granzyme A, B and C genes in the CD8+ T-cells. This increase expression causes the CD8+ T-cells to mature into Cytoxic T Lymphocytes (CTLs). The exogenous IL-2 is required for the granzyme proteins. As stated previously, CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Once in the tumor cell, these enzymes are able to breakup (lyse) the cell and destroy it. This is the beginning of the end for the cancer cells.
Conclusion:
The devil is in the details!!!
Interluekin-2 concentration and timing of the addition of the exogenous IL-2 has a critical role to play in The Orchestration of an Inmmune Response.
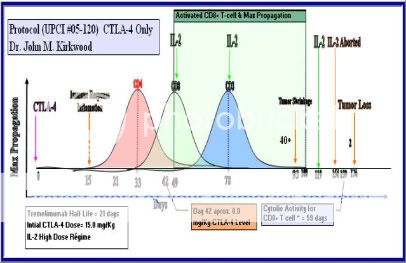
Lastly, I am going to leave you with my graphical representation of my therapy that has save my life. Study it. I willing to bet that this graph is medical history in the making base on the science research that I did.
Reference
1. Opposing Effects of IL-2 in Tumor Immunotherapy: Promoting CD8 T Cell Growth and Inducing Apoptosis1
Protul Shrikant2 and Matthew F. Mescher3
Center for Immunology, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN 55455 The Journal of Immunology, 2002, 169: 1753-1759.
http://www.jimmunol.org/cgi/content/full/169/4/1753
Take Care,
Jimmy B

















