Yes!!! I have blueberries three time a week on me morning cereal. Yes, I also Juice 5 days week. Weekends I have pomagranite/blueberry juice.
See, I believe we have to help those little T-cells and the whole immune system get some good eats, to keep the immune system.
Title: Can Blueberries Really Shrink Tumors And Protect Us From Cancer
» Posted: February 17th, 2009
» Author: NatureDoc
» Filed Under: Breast Cancer, Cancer, Foods That Heal, Herbal Remedies For Diabetes
Blueberries are indeed one of the “super foods” that have been placed on the earth by the Creator! They are a major source of flavonoids, in particular anthocyanins and flavanols.
Blueberries are a good source of vitamin C and they also contain vitamin A, iron, potassium and magnesium.
Blueberries are also thought to be helpful in improving memory function and healthy aging. In fact, they have been shown to cross the blood brain barrier after dietary intake; notwithstanding the precise mechanisms by which these plant-derived molecules affect the brain are unknown.
Even Blueberry leaves contain polyphenols that demonstrate alpha-amylase inhibitor activity and may represent an encouraging adjunctive treatment for type 2 diabetes.
Blueberries And Tumors
Notwithstanding all of the amazing benefits of the blueberry; we want to turn our attention to the promising effects this humble, but powerful, berry may have on tumors, and especially tumors in children.
Pointing to a recent study involving the blueberry; health researcher, John Barron, writes, “The latest news comes from a study out of Ohio State University finding that blueberries may shrink blood vessel tumors in babies while doubling survival rates. The research team fed blueberry extract to mice that had tumors. The mice receiving the extract lived twice as long as those mice that had blood vessel tumors but didn’t take the extract. Also, the tumors in the blueberry mice were 60 percent smaller than those in the control group.”
Source:http://phase3ministries.com/wordpress/2009/02/17/can-blueberries-really-shrink-tumors-and-protect-us-from-cancer/
Can Blueberries Really Shrink Tumors And Protect Us From Cancer
Take care
Jimmy B
This is Jim Breitfeller's journey into the Maze of Melanoma. Jim Breitfeller has gathered medical information for the patient and the caregiver. As Lance Armstrong would say "Lets stand Up to Cancer" Jim's Battle with the Beast July 2005 to present.
Saturday, February 28, 2009
Friday, February 27, 2009
Cancer Miracles !!!!!!! Melanoma Jim Breitfeller
HEALTH
Medical mysteries
Last Updated: Friday, February 27, 2009
Cancer miracles
By Robert Langreth, Forbes.com
Charles Burrows noticed a strange lump on his stomach in the summer of 2005. By November the pain was so bad it felt like a knife was stabbing him in the stomach. A ct scan and a biopsy confirmed Burrows' worst fears: He had inoperable liver cancer.
Few cancers have a worse prognosis. His tumor, the size of a baseball, was already starting to strangle the portal vein going into the liver. Doctors at the Phoenix Veterans Affairs Health Care System told Burrows, then 56 years old, there was nothing they could do. "They said, 'Get your affairs in order because you have 30 days to live, maybe 60,'" recalls Burrows, who is divorced with three grown kids.
Burrows quit his carpentry job and spent the next two months in a fog. Then things got very strange. In February 2006 Burrows developed abdominal bloating, shaking, chills and nausea. Soon after that he noticed that the lump on his stomach was gone. By then his daughter had found a doctor in private practice willing to consider treating him. But the doctor couldn't find a tumor. He went back to the VA, where gastroenterologist Nooman Gilani was flabbergasted when computed tomography and magnetic resonance imaging scans showed no sign of cancer. Where the tumor had once been, there was "literally empty space," Gilani says.
Burrows remains free of cancer three years later and still seems dazed by the turn of events. "I won a lottery, and I don't understand why," he says. "I would like someone to explain to me what the heck happened."
And there is more!!!!!
Source:http://www.cbc.ca/health/story/2009/02/25/f-forbes-cancer.html
Cancer Miracles
Take Care
Have a great weekend!!!!!
Jimmy B
Medical mysteries
Last Updated: Friday, February 27, 2009
Cancer miracles
By Robert Langreth, Forbes.com
Charles Burrows noticed a strange lump on his stomach in the summer of 2005. By November the pain was so bad it felt like a knife was stabbing him in the stomach. A ct scan and a biopsy confirmed Burrows' worst fears: He had inoperable liver cancer.
Few cancers have a worse prognosis. His tumor, the size of a baseball, was already starting to strangle the portal vein going into the liver. Doctors at the Phoenix Veterans Affairs Health Care System told Burrows, then 56 years old, there was nothing they could do. "They said, 'Get your affairs in order because you have 30 days to live, maybe 60,'" recalls Burrows, who is divorced with three grown kids.
Burrows quit his carpentry job and spent the next two months in a fog. Then things got very strange. In February 2006 Burrows developed abdominal bloating, shaking, chills and nausea. Soon after that he noticed that the lump on his stomach was gone. By then his daughter had found a doctor in private practice willing to consider treating him. But the doctor couldn't find a tumor. He went back to the VA, where gastroenterologist Nooman Gilani was flabbergasted when computed tomography and magnetic resonance imaging scans showed no sign of cancer. Where the tumor had once been, there was "literally empty space," Gilani says.
Burrows remains free of cancer three years later and still seems dazed by the turn of events. "I won a lottery, and I don't understand why," he says. "I would like someone to explain to me what the heck happened."
And there is more!!!!!
Source:http://www.cbc.ca/health/story/2009/02/25/f-forbes-cancer.html
Cancer Miracles
Take Care
Have a great weekend!!!!!
Jimmy B
Hand and Hand Tumor Rejection ..Melanoma ..Jim Breitfeller
This diagram goes with the "Tumor Rejection Piece"

Tumor Rejection!!!!!!
To cause the rejection of the tumors cells, The immune system must orchestrate a chain of events mediated by several types of Leukocytes including Dendtric cells (DC), Natuaral Killer cells (NK), CD4+ and CD8+ lymphocytes and others. This orchestration has many players in it including the T-Regs, sercreted cytokines, and Monoclonal antibodies (mAb’s) and complexes. It is a delicate balance between self and non-self.
At the Lymph Node drainage area, the lymphocytes (CD4+ and CD8+) with their T-cell receptors (TCRs) are able to scan the Dendtric cells (DC’s) for antigen-MHC molecules. (ag-MHC) major histocompatability complex (class I or II). Based on the two signal model, a second signal from CD28 molecule is needed to activate the T-cells (T-lymphocytes). If communcation breaksdown, and the TCR signal only happens, it can lead to tolerance by means of fuctional paralysis of the (APCs) Antigen presenting cells (Anergy) or by the induction of clonal deletion (apoptosis). Anergic cells can act as regulatory T cells by competing at the sites of antigen presentation and adsorbing out stimulatory cytokines such as IL-2. This can halt the activation of the T- lymphocytes and no immune response is initiated.
Once activated fully, the CD4+ T-cells can mobilize to where the event will take place and usually sends out a “danger signal” inflammation. The activated CD4+ T-cells can secrete many different cytokines including IL-4, IL-2 and activate the TH2 cells which are a subset of the CD4+ cells. The TH2 cells stimulate the B cells to mature into plasma cells that secrete antibodies. These antibodies that are produced are the cell-destructive kinds that have anti-tumor behavior. The CD4+ can also cross-prime CD8+ T-cells in the presence of IL-2 and are called (CTLs)Cytotoxic T Lymphocytes. Cross-priming is another name for cross-presentation. The role of the CD8+ T cells is to monitor all the cells of the body, ready to destroy any that express foreign antigen fragments in their class I molecules.
Some CD4+ T cells can develop into CTLs, but they can attack only those cell types (e.g. B cells, macrophages, dendritic cells) that express class II MHC molecules. Virtually every cell in the body expresses class I MHC molecules, so CD8+ CTLs are not limited in the targets they can attack. CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. When the CTL binds to its target, the contents of the granules are discharged. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Granzymes are serine proteases. The serine proteases are a family of enzymes that cut certain bonds in other proteins. It is similar to what is in your laundry detergent. They are known as detergent enzymes. They break the bond between the dirt and the fabric. By breaking up these proteins, they start destroying the intracellular workings of the tumor cells.
Next Piece is how the tumor protects itself from the Killer T-cells through a microenviroment and Tregs.
Just plugging away!!
Jimmy B

Tumor Rejection!!!!!!
To cause the rejection of the tumors cells, The immune system must orchestrate a chain of events mediated by several types of Leukocytes including Dendtric cells (DC), Natuaral Killer cells (NK), CD4+ and CD8+ lymphocytes and others. This orchestration has many players in it including the T-Regs, sercreted cytokines, and Monoclonal antibodies (mAb’s) and complexes. It is a delicate balance between self and non-self.
At the Lymph Node drainage area, the lymphocytes (CD4+ and CD8+) with their T-cell receptors (TCRs) are able to scan the Dendtric cells (DC’s) for antigen-MHC molecules. (ag-MHC) major histocompatability complex (class I or II). Based on the two signal model, a second signal from CD28 molecule is needed to activate the T-cells (T-lymphocytes). If communcation breaksdown, and the TCR signal only happens, it can lead to tolerance by means of fuctional paralysis of the (APCs) Antigen presenting cells (Anergy) or by the induction of clonal deletion (apoptosis). Anergic cells can act as regulatory T cells by competing at the sites of antigen presentation and adsorbing out stimulatory cytokines such as IL-2. This can halt the activation of the T- lymphocytes and no immune response is initiated.
Once activated fully, the CD4+ T-cells can mobilize to where the event will take place and usually sends out a “danger signal” inflammation. The activated CD4+ T-cells can secrete many different cytokines including IL-4, IL-2 and activate the TH2 cells which are a subset of the CD4+ cells. The TH2 cells stimulate the B cells to mature into plasma cells that secrete antibodies. These antibodies that are produced are the cell-destructive kinds that have anti-tumor behavior. The CD4+ can also cross-prime CD8+ T-cells in the presence of IL-2 and are called (CTLs)Cytotoxic T Lymphocytes. Cross-priming is another name for cross-presentation. The role of the CD8+ T cells is to monitor all the cells of the body, ready to destroy any that express foreign antigen fragments in their class I molecules.
Some CD4+ T cells can develop into CTLs, but they can attack only those cell types (e.g. B cells, macrophages, dendritic cells) that express class II MHC molecules. Virtually every cell in the body expresses class I MHC molecules, so CD8+ CTLs are not limited in the targets they can attack. CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. When the CTL binds to its target, the contents of the granules are discharged. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Granzymes are serine proteases. The serine proteases are a family of enzymes that cut certain bonds in other proteins. It is similar to what is in your laundry detergent. They are known as detergent enzymes. They break the bond between the dirt and the fabric. By breaking up these proteins, they start destroying the intracellular workings of the tumor cells.
Next Piece is how the tumor protects itself from the Killer T-cells through a microenviroment and Tregs.
Just plugging away!!
Jimmy B
Thursday, February 26, 2009
Jay Tenenbaum Urges Collaboration To Treat the Long Tail of Disease Melanoma..Jim Breitfeller
By Kevin Davies
February 26, 2009 SAN FRANCISCO—In the powerful opening keynote at CHI’s Molecular Medicine Tri-Conference on Wednesday, Jay “Marty” Tenenbaum, founder and chairman of CollabRx, urged members of the life sciences community to share their resources to empower personalized research and help satisfy the unmet medical needs of the “long tail” of disease. “As a patient… I want to tap all of the world’s knowledge and all of the world’s resources into curing my disease,” Tenenbaum said.
Tenenbaum, a highly successful Internet entrepreneur in the 1990s, is a cancer survivor. Ten years ago, suffering from metastatic melanoma, he was given 12 months to live. He researched various experimental drug treatments, and credits a failed cancer vaccine, among other drugs, for saving his life. Through the company he founded, CollabRx, Tenenbaum aims to leverage the extraordinary untapped expertise and resources across the industry to empower individual patient healthcare through personalized research.
This sounds Familiar!!!!!!!!!
Source:http://www.bio-itworld.com/2009/02/26/tenenbaum-mmtc-keynote.html
Jay Tenenbaum Urges Collaboration To Treat the Long Tail of Disease
http://podcast.mktw.net/wsj/audio/20080728/pod-wsjmarcus/pod-wsjmarcus.mp3
Jay Tenenbaum Urges Collaboration Podcast
Take care
Jimmy B
February 26, 2009 SAN FRANCISCO—In the powerful opening keynote at CHI’s Molecular Medicine Tri-Conference on Wednesday, Jay “Marty” Tenenbaum, founder and chairman of CollabRx, urged members of the life sciences community to share their resources to empower personalized research and help satisfy the unmet medical needs of the “long tail” of disease. “As a patient… I want to tap all of the world’s knowledge and all of the world’s resources into curing my disease,” Tenenbaum said.
Tenenbaum, a highly successful Internet entrepreneur in the 1990s, is a cancer survivor. Ten years ago, suffering from metastatic melanoma, he was given 12 months to live. He researched various experimental drug treatments, and credits a failed cancer vaccine, among other drugs, for saving his life. Through the company he founded, CollabRx, Tenenbaum aims to leverage the extraordinary untapped expertise and resources across the industry to empower individual patient healthcare through personalized research.
This sounds Familiar!!!!!!!!!
Source:http://www.bio-itworld.com/2009/02/26/tenenbaum-mmtc-keynote.html
Jay Tenenbaum Urges Collaboration To Treat the Long Tail of Disease
http://podcast.mktw.net/wsj/audio/20080728/pod-wsjmarcus/pod-wsjmarcus.mp3
Jay Tenenbaum Urges Collaboration Podcast
Take care
Jimmy B
Labels:
molecular profiling,
Personalized Medicine,
Vaccine
Fewer, Smaller Skin Cancer Tumors After Blocking Protein Melanoma ..Jim Breitfeller
Main Category: Melanoma / Skin Cancer
Also Included In: Biology / Biochemistry; Vascular; Dermatology
Article Date: 18 Feb 2009 - 1:00 PST
New research suggests that blocking the activity of a protein in the blood could offer powerful protection against some skin cancers.
In the study, normal mice and mice that had a genetically engineered protein deficiency were exposed to almost a year of ultraviolet light that mimics chronic sun exposure. The mice that lacked the protein developed fewer, smaller, less aggressive and less vascular skin cancer tumors than did the normal mice.
Because a low-dose drug that blocks the protein's activity in the blood is currently under investigation by a Pennsylvania pharmaceutical company, the researchers hope that someday, a simple pill might help prevent or treat nonmelanoma skin cancer in people at highest risk for the disease.
Source:http://www.medicalnewstoday.com/articles/139335.php?nfid=76490
Also Included In: Biology / Biochemistry; Vascular; Dermatology
Article Date: 18 Feb 2009 - 1:00 PST
New research suggests that blocking the activity of a protein in the blood could offer powerful protection against some skin cancers.
In the study, normal mice and mice that had a genetically engineered protein deficiency were exposed to almost a year of ultraviolet light that mimics chronic sun exposure. The mice that lacked the protein developed fewer, smaller, less aggressive and less vascular skin cancer tumors than did the normal mice.
Because a low-dose drug that blocks the protein's activity in the blood is currently under investigation by a Pennsylvania pharmaceutical company, the researchers hope that someday, a simple pill might help prevent or treat nonmelanoma skin cancer in people at highest risk for the disease.
Source:http://www.medicalnewstoday.com/articles/139335.php?nfid=76490
Wednesday, February 25, 2009
In Loving Memory ..Melanoma.. Jim Breitfeller
Donna R. Di Ambrosia
August 25, 1937 – February 23, 2009
Donna R. Di Ambrosia, resident of Downey California for 60 years, passed away on February 23, 2009, after a 17 month long battle with Cancer.
"She was born on August 25, 1937 at Doctors Hospital in Los Angeles, to William and Helen May. She graduated from Downey High School in 1955 and attended Compton and Fullerton Junior Colleges. She worked for Lockheed and then Ford Motor Company in 1958 where she met her loving husband Kenneth. They were together for 50 years, spending 45 years as husband and wife.
Donna is survived by her husband Kenny, her two daughters, Gina and Lisa, her son-in-laws, Gary and John and her granddaughters, Kayla and Alyssa.
In lieu of flowers, the family requests a donation in the name of Donna Di Ambrosia be made to the Melanoma Research Foundation at www.melanoma.org."
Please stop by her carepage
Thanks.
cp:DonnaDia
August 25, 1937 – February 23, 2009
Donna R. Di Ambrosia, resident of Downey California for 60 years, passed away on February 23, 2009, after a 17 month long battle with Cancer.
"She was born on August 25, 1937 at Doctors Hospital in Los Angeles, to William and Helen May. She graduated from Downey High School in 1955 and attended Compton and Fullerton Junior Colleges. She worked for Lockheed and then Ford Motor Company in 1958 where she met her loving husband Kenneth. They were together for 50 years, spending 45 years as husband and wife.
Donna is survived by her husband Kenny, her two daughters, Gina and Lisa, her son-in-laws, Gary and John and her granddaughters, Kayla and Alyssa.
In lieu of flowers, the family requests a donation in the name of Donna Di Ambrosia be made to the Melanoma Research Foundation at www.melanoma.org."
Please stop by her carepage
Thanks.
cp:DonnaDia
IL-2 Regulates Perforin and Granzyme Gene Expression in CD8+ T Cells Independently ..Melanoma.. Jim Breitfeller
IL-2 Regulates Perforin and Granzyme Gene Expression in CD8+ T Cells Independently of Its Effects on Survival and Proliferation
I know this doesn't sound exciting to you, but it does to me. See I am slowly piecing the puzzle together that made my Sequential Cancer Treatment work so far.If I knew how complex the biochemistry of our immune system was, I would asked for cliff notes.
"Granule-mediated cytotoxicity is one of the major mechanisms used by CD8+ T cells to eliminate harmful or foreign bodies, such as virus-infected cells, tumors, and allografts. After Ag recognition, activated CD8+ T cells release the contents of their cytotoxic granules into the extracellular space, where they are taken up by the target cell, and apoptosis is initiated (1). The cytotoxic granules contain a number of molecules, including the pore-forming protein, perforin, and serine proteases, known as granzymes. Perforin was originally thought to cause cell lysis by penetrating the target cell membrane (2), but recent work favors the theory that perforin functions by enabling the granzymes to escape from endosomes into the cytosol of the target cell (3, 4). Whatever its exact role, perforin is essential, because Ag-specific granule-mediated cytotoxicity is absent in perforin-deficient CD8+ T cells and NK cells (5)."
I think I can do some hand waving but I still need to know how my immune system was able to differentiate between self (the body) and non-self (the tumor). I believe one of the keys may be the T-reg cells.
"These studies suggest that IL-2 itself may either directly or indirectly play an important role in the development and/or function of this unique population of CD25+ suppressor cells."
Source:http://jem.rupress.org/cgi/content/full/188/2/287
CD4+CD25+ Immunoregulatory T Cells Suppress Polyclonal T Cell Activation In Vitro by Inhibiting Interleukin 2 Production By Angela M. Thornton and Ethan M. Shevach
"Regulatory T cells (sometimes known as suppressor T cells) are a specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to self-antigens. The existence of a dedicated population of suppressive T cells was the subject of significant controversy among immunologists for many years. However, recent advances in the molecular characterization of this cell population have firmly established their existence and their critical role in the vertebrate immune system. Interest in regulatory T cells has been heightened by evidence from experimental mouse models demonstrating that the immunosuppressive potential of these cells can be harnessed therapeutically to treat autoimmune diseases and facilitate transplantation tolerance or specifically eliminated to potentiate cancer immunotherapy.
T regulatory cell populations
T regulatory cells are a component of the immune system that suppress immune responses of other cells. This is an important "self-check" built into the immune system so that responses do not go haywire. Regulatory T cells come in many forms, including those that express the CD8 transmembrane glycoprotein (CD8+ T cells), those that express CD4, CD25 and Foxp3 (CD4+CD25+ regulatory T cells or "Tregs") and other T cell types that have suppressive function. These cells are involved in closing down immune responses after they have successfully tackled invading organisms, and also in keeping in check immune responses that may potentially attack one's own tissues (autoimmunity).
CD4+Foxp3+ regulatory T cells have been referred to as "naturally-occurring" regulatory T cells to distinguish them from "suppressor" T cell populations that are generated in vitro. The regulatory T cell field is further complicated by reports of additional suppressive T cell populations, including Tr1, CD8+CD28-, and Qa-1 restricted T cells. However the contribution of these populations to self-tolerance and immune homeostasis is less well defined."
Source:Wikipedia
Back to the books
I know this doesn't sound exciting to you, but it does to me. See I am slowly piecing the puzzle together that made my Sequential Cancer Treatment work so far.If I knew how complex the biochemistry of our immune system was, I would asked for cliff notes.
"Granule-mediated cytotoxicity is one of the major mechanisms used by CD8+ T cells to eliminate harmful or foreign bodies, such as virus-infected cells, tumors, and allografts. After Ag recognition, activated CD8+ T cells release the contents of their cytotoxic granules into the extracellular space, where they are taken up by the target cell, and apoptosis is initiated (1). The cytotoxic granules contain a number of molecules, including the pore-forming protein, perforin, and serine proteases, known as granzymes. Perforin was originally thought to cause cell lysis by penetrating the target cell membrane (2), but recent work favors the theory that perforin functions by enabling the granzymes to escape from endosomes into the cytosol of the target cell (3, 4). Whatever its exact role, perforin is essential, because Ag-specific granule-mediated cytotoxicity is absent in perforin-deficient CD8+ T cells and NK cells (5)."
I think I can do some hand waving but I still need to know how my immune system was able to differentiate between self (the body) and non-self (the tumor). I believe one of the keys may be the T-reg cells.
"These studies suggest that IL-2 itself may either directly or indirectly play an important role in the development and/or function of this unique population of CD25+ suppressor cells."
Source:http://jem.rupress.org/cgi/content/full/188/2/287
CD4+CD25+ Immunoregulatory T Cells Suppress Polyclonal T Cell Activation In Vitro by Inhibiting Interleukin 2 Production By Angela M. Thornton and Ethan M. Shevach
"Regulatory T cells (sometimes known as suppressor T cells) are a specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to self-antigens. The existence of a dedicated population of suppressive T cells was the subject of significant controversy among immunologists for many years. However, recent advances in the molecular characterization of this cell population have firmly established their existence and their critical role in the vertebrate immune system. Interest in regulatory T cells has been heightened by evidence from experimental mouse models demonstrating that the immunosuppressive potential of these cells can be harnessed therapeutically to treat autoimmune diseases and facilitate transplantation tolerance or specifically eliminated to potentiate cancer immunotherapy.
T regulatory cell populations
T regulatory cells are a component of the immune system that suppress immune responses of other cells. This is an important "self-check" built into the immune system so that responses do not go haywire. Regulatory T cells come in many forms, including those that express the CD8 transmembrane glycoprotein (CD8+ T cells), those that express CD4, CD25 and Foxp3 (CD4+CD25+ regulatory T cells or "Tregs") and other T cell types that have suppressive function. These cells are involved in closing down immune responses after they have successfully tackled invading organisms, and also in keeping in check immune responses that may potentially attack one's own tissues (autoimmunity).
CD4+Foxp3+ regulatory T cells have been referred to as "naturally-occurring" regulatory T cells to distinguish them from "suppressor" T cell populations that are generated in vitro. The regulatory T cell field is further complicated by reports of additional suppressive T cell populations, including Tr1, CD8+CD28-, and Qa-1 restricted T cells. However the contribution of these populations to self-tolerance and immune homeostasis is less well defined."
Source:Wikipedia
Back to the books
Labels:
CD8+T cells,
Granzyme,
IL-2,
immune system,
Immunosuppressive Activity,
Melanoma,
My Theory,
Perorin,
tregs
I AM STUCK AT BASE CAMP 2!!! Melanoma Jim Breitfeller
I am stuck at base camp 2 waiting for the weather to clear.
See, I am trying to get my arms around T-regs and the tumor's microenviroment. And How the addition of IL-2 played into the tumor's demise. I am scouring the research papers and I alread have printed out one reem of paper and started the second. Nothing like digging in for the day, week or?? So my accent on Mt. Everest is on hold until I find the right path and the enviromental conditions change.


Take care
Jimmy B
See, I am trying to get my arms around T-regs and the tumor's microenviroment. And How the addition of IL-2 played into the tumor's demise. I am scouring the research papers and I alread have printed out one reem of paper and started the second. Nothing like digging in for the day, week or?? So my accent on Mt. Everest is on hold until I find the right path and the enviromental conditions change.


Take care
Jimmy B
Are You Going to Finish Strong? Melanoma Jim Breitfeller
Sometimes, I get to technical and I may seem to be preaching. I guess what I am tring to say is never give up. You can do it.
Please check out this video. You can Climb Mount Everest!!!!!!!!
http://www.maniacworld.com/are-you-going-to-finish-strong.html
Are You Going to Finish Strong?
We are all in it together!!!
We are going to finnish STRONG!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Take care
Please check out this video. You can Climb Mount Everest!!!!!!!!
http://www.maniacworld.com/are-you-going-to-finish-strong.html
Are You Going to Finish Strong?
We are all in it together!!!
We are going to finnish STRONG!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Take care
Monday, February 23, 2009
Patient knowledge of health information influences cancer treatment Melanoma Jim Breitfeller
Date:2/22/2009
"In their review, authors led by Stacy Gray, M.D. of the Dana-Farber Cancer Institute in Boston note that in the last several decades, patients have become more involved in their health care as patient autonomy has become increasingly important. That change has been accompanied by unprecedented growth in the amount of health information available to patients. Studies show nearly four out of ten of cancer patients seek cancer information on the internet. But the authors say it is unclear how these phenomena influence a cancer patient's treatment."
Source:http://www.bio-medicine.org/medicine-news-1/Patient-knowledge-of-health-information-influences-cancer-treatment-37343-1/
Patient knowledge of health information influences cancer treatment
You can influence your Path forward if you take the time to do the Research/HOMEWORK.
Jimmy B
"In their review, authors led by Stacy Gray, M.D. of the Dana-Farber Cancer Institute in Boston note that in the last several decades, patients have become more involved in their health care as patient autonomy has become increasingly important. That change has been accompanied by unprecedented growth in the amount of health information available to patients. Studies show nearly four out of ten of cancer patients seek cancer information on the internet. But the authors say it is unclear how these phenomena influence a cancer patient's treatment."
Source:http://www.bio-medicine.org/medicine-news-1/Patient-knowledge-of-health-information-influences-cancer-treatment-37343-1/
Patient knowledge of health information influences cancer treatment
You can influence your Path forward if you take the time to do the Research/HOMEWORK.
Jimmy B
Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment Melanoma Jim Breitfeller
Thomas F. Gajewski
Author's Affiliation: Departments of Pathology and Medicine, University of Chicago, Chicago, Illinois
Requests for reprints: Thomas F. Gajewski, University of Chicago, 5841 South Maryland Avenue, MC2115, Chicago, IL 60637. Phone: 773-702-4601; Fax: 773-702-3163; E-mail: tgajewsk@medicine.bsd.uchicago.edu.
"Metastatic melanoma tumors seem to be deficient in expression of B7-1 and B7-2 (16), which are important costimulatory factors for full T-cell activation (17). Stimulation through the T-cell antigen receptor without B7 costimulation has been shown to induce a hyporesponsive state termed anergy (18, 19). In support of this possibility, we recently have observed in a mouse model that a T-cell hyporesponsive state consistent with anergy occurs in mice bearing B7-negative tumors (20). In this situation, tumor rejection indeed can occur when B7-1 is transfected into the tumor cells, an observation that has been reported by multiple laboratories in other model systems (21, 22)."
In my case we stimulated the B7 receptor with anti-CTLA-4 Blockage, in the present of the antigen presenting cell (APC) that activated the CD4+T-cell it was allowed to propagate for 49 days (43 days gave the maximum propagation. Than we added the IL-2 therapy which we know by experiments the done by Rosenberg that Il-2 is able to pass throught the Tumor's microenvironment.
In 1980, Dr. Steven A. Roesnberg and colleagues discovered novel novel method for killing metastatic cancer cells. They took lymphoid cells and exposed them to interluekin-2 (IL-2).These cells were able to lyse the tumor cells and kill them. The were a different population than the Natural Killer cells. They coined the term “Lymphokine-activated killer cells” (LAK) for short.
Also, When patients do IL-2 therapy, they have side-effect Known as cell leakage. Il-2 makes the cell wall porous.
With the tumor cells walls compromised and with the activated effector T cells, the assualt on the tumors begin.
At Least, that is the way I envision it. There is probably a lot more going on behind the scenes.
Jimmy B
Author's Affiliation: Departments of Pathology and Medicine, University of Chicago, Chicago, Illinois
Requests for reprints: Thomas F. Gajewski, University of Chicago, 5841 South Maryland Avenue, MC2115, Chicago, IL 60637. Phone: 773-702-4601; Fax: 773-702-3163; E-mail: tgajewsk@medicine.bsd.uchicago.edu.
"Metastatic melanoma tumors seem to be deficient in expression of B7-1 and B7-2 (16), which are important costimulatory factors for full T-cell activation (17). Stimulation through the T-cell antigen receptor without B7 costimulation has been shown to induce a hyporesponsive state termed anergy (18, 19). In support of this possibility, we recently have observed in a mouse model that a T-cell hyporesponsive state consistent with anergy occurs in mice bearing B7-negative tumors (20). In this situation, tumor rejection indeed can occur when B7-1 is transfected into the tumor cells, an observation that has been reported by multiple laboratories in other model systems (21, 22)."
In my case we stimulated the B7 receptor with anti-CTLA-4 Blockage, in the present of the antigen presenting cell (APC) that activated the CD4+T-cell it was allowed to propagate for 49 days (43 days gave the maximum propagation. Than we added the IL-2 therapy which we know by experiments the done by Rosenberg that Il-2 is able to pass throught the Tumor's microenvironment.
In 1980, Dr. Steven A. Roesnberg and colleagues discovered novel novel method for killing metastatic cancer cells. They took lymphoid cells and exposed them to interluekin-2 (IL-2).These cells were able to lyse the tumor cells and kill them. The were a different population than the Natural Killer cells. They coined the term “Lymphokine-activated killer cells” (LAK) for short.
Also, When patients do IL-2 therapy, they have side-effect Known as cell leakage. Il-2 makes the cell wall porous.
With the tumor cells walls compromised and with the activated effector T cells, the assualt on the tumors begin.
At Least, that is the way I envision it. There is probably a lot more going on behind the scenes.
Jimmy B
Labels:
CD4+ Tcells,
CTLA-4,
Dr. Gajewski,
IL-2,
Rosenberg,
t,
Tumor Elimination
Sunday, February 22, 2009
Here Goes!!! I am going to Spill the Beans!!!!!! Melanoma Jim Breitfeller
"The induction of an immunological antitumor response capable of eradicating metastatic tumors is the ultimate goal of immunotherapy. We have recently shown that this can be achieved by interleukin 2 (IL-2) therapy directed to the tumor microenvironment by a recombinant antibody–IL-2 fusion protein. It is not known, however, whether this curative treatment is associated with a predominance of T cells carrying specific T cell receptor variable ? regions (TCRBV) or the presence of clonally expanded T cells."
Activation of preexisting T cell clones by targeted interleukin 2 therapy
Per thor Straten,* Per Guldberg,* Tina Seremet,* Ralph A. Reisfeld,†‡ Jesper Zeuthen,* and Jürgen C. Becker§
*Department of Tumor Cell Biology, Division of Cancer Biology, Danish Cancer Society, DK-2100 Copenhagen, Denmark; †Department of Immunology, Scripps Research Institute, La Jolla, CA 92037; and §Department of Dermatology, School of Medicine, D-97080 Würzburg, Germany
‡To whom reprint requests should be addressed at: Department of Immunology, Scripps Research Institute, IMM 13, R218, 10550 North Torrey Pines Road, La Jolla, CA 92037.
Communicated by Frank J. Dixon, Scripps Research Institute, La Jolla, CA
Received February 19, 1998; Accepted April 30, 1998.
"Melanoma is a highly malignant tumor but several lines of evidence suggest that it is capable of eliciting a specific immune response, i.e., a number of melanoma-associated antigens have been identified and the presence of clonotypic T cells has been demonstrated in melanoma lesions (1–4). Therefore, several immunomodulatory therapeutic approaches were initiated to improve the prognosis of melanoma patients. Interleukin 2 (IL-2) is one of the most potent antitumor cytokines known quote (5), and was recently approved for treatment of metastatic melanoma. However, objective responses induced by systemic IL-2 therapy are still insufficient, and the associated side effects are severe (6). These findings are due to the fact that a systemic application of IL-2 disregards the paracrine nature of this cytokine under physiological conditions (7).
As a means to target IL-2 directly to the tumor site, we have recently shown that human IL-2 can be genetically engineered as a fusion protein with the chimeric mouse–human mAb 14.18 which recognizes the ganglioside GD2, retaining both antigen binding and cytokine activity (8).
Furthermore, we have shown that treatment with this antibody–IL-2 fusion protein can eradicate human hepatic and pulmonary melanoma metastases in severe combined immunodeficient mice (9) as well as autologous murine B16 melanomas (10). Although it was shown in these studies that tumor eradication was dependent on CD8+ T cells, it is not known whether tumor clearance is associated with a clonal expansion of T cells. Furthermore, it remains to be established whether such a clonal expansion would be due to a de novo induction or to the activation and expansion of preexisting T cell clones. Here, we demonstrate both the overexpression of certain T cell receptor variable β regions (TCRBV) as well as the clonal expansion of T cells in melanoma lesions subsequent to targeted IL-2 therapy.
However, clonally expanded T cells were also detectable prior to the
thrapy, suggesting that antibody–IL-2-targeted therapy acts as an activator rather than an inducer of an antitumor T cell response."
Source:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=21154
Activation of preexisting T cell clones by targeted interleukin 2 therapy
"Melanoma and the Magic Bullet (Monoclonal Antibodies)"
You have read it here First!!!!!!!!!
I still have write the IL-2 part, But I believe the concept is all there.
Now I have to work on the protocol of the "Antibody-IL-2 therapy"
That is why my therapy "Antibody-IL-2 therapy" worked!!!!!!!!
I can smell the sweet Success!!!!!!!

Jimmy B
Activation of preexisting T cell clones by targeted interleukin 2 therapy
Per thor Straten,* Per Guldberg,* Tina Seremet,* Ralph A. Reisfeld,†‡ Jesper Zeuthen,* and Jürgen C. Becker§
*Department of Tumor Cell Biology, Division of Cancer Biology, Danish Cancer Society, DK-2100 Copenhagen, Denmark; †Department of Immunology, Scripps Research Institute, La Jolla, CA 92037; and §Department of Dermatology, School of Medicine, D-97080 Würzburg, Germany
‡To whom reprint requests should be addressed at: Department of Immunology, Scripps Research Institute, IMM 13, R218, 10550 North Torrey Pines Road, La Jolla, CA 92037.
Communicated by Frank J. Dixon, Scripps Research Institute, La Jolla, CA
Received February 19, 1998; Accepted April 30, 1998.
"Melanoma is a highly malignant tumor but several lines of evidence suggest that it is capable of eliciting a specific immune response, i.e., a number of melanoma-associated antigens have been identified and the presence of clonotypic T cells has been demonstrated in melanoma lesions (1–4). Therefore, several immunomodulatory therapeutic approaches were initiated to improve the prognosis of melanoma patients. Interleukin 2 (IL-2) is one of the most potent antitumor cytokines known quote (5), and was recently approved for treatment of metastatic melanoma. However, objective responses induced by systemic IL-2 therapy are still insufficient, and the associated side effects are severe (6). These findings are due to the fact that a systemic application of IL-2 disregards the paracrine nature of this cytokine under physiological conditions (7).
As a means to target IL-2 directly to the tumor site, we have recently shown that human IL-2 can be genetically engineered as a fusion protein with the chimeric mouse–human mAb 14.18 which recognizes the ganglioside GD2, retaining both antigen binding and cytokine activity (8).
Furthermore, we have shown that treatment with this antibody–IL-2 fusion protein can eradicate human hepatic and pulmonary melanoma metastases in severe combined immunodeficient mice (9) as well as autologous murine B16 melanomas (10). Although it was shown in these studies that tumor eradication was dependent on CD8+ T cells, it is not known whether tumor clearance is associated with a clonal expansion of T cells. Furthermore, it remains to be established whether such a clonal expansion would be due to a de novo induction or to the activation and expansion of preexisting T cell clones. Here, we demonstrate both the overexpression of certain T cell receptor variable β regions (TCRBV) as well as the clonal expansion of T cells in melanoma lesions subsequent to targeted IL-2 therapy.
However, clonally expanded T cells were also detectable prior to the
thrapy, suggesting that antibody–IL-2-targeted therapy acts as an activator rather than an inducer of an antitumor T cell response."
Source:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=21154
Activation of preexisting T cell clones by targeted interleukin 2 therapy
"Melanoma and the Magic Bullet (Monoclonal Antibodies)"
You have read it here First!!!!!!!!!
I still have write the IL-2 part, But I believe the concept is all there.
Now I have to work on the protocol of the "Antibody-IL-2 therapy"
That is why my therapy "Antibody-IL-2 therapy" worked!!!!!!!!
I can smell the sweet Success!!!!!!!

Jimmy B
Saturday, February 21, 2009
Next Slide Please!!!!!!! Melanoma Jim Breitfeller
BobbyO L, This Slide for You!!!!!!

Follow the "YELLOW BRICK ROAD!!!!!"
Cell-mediated immunity is an immune response that does not involve antibodies or complement but rather involves the activation of macrophages, natural killer cells (NK), antigen-specific cytotoxic T-lymphocytes (CTL), and the release of various cytokines in response to an antigen. Historically, the immune system was separated into two branches: humoral immunity, for which the protective function of immunization could be found in the humor (cell-free bodily fluid or serum) and cellular immunity, for which the protective function of immunization was associated with cells. CD4 cells or helper T cells provide protection against different pathogens.
Cellular immunity protects the body by:
1. activating antigen-specific cytotoxic T-lymphocytes(CTL)that are able to induce apoptosis in body cells displaying epitopes of foreign antigen on their surface, such as virus-infected cells, cells with intracellular bacteria, and cancer cells displaying tumor antigens;
2. activating macrophages and natural killer cells, enabling them to destroy intracellular pathogens; and
3. stimulating cells to secrete a variety of cytokines that influence the function of other cells involved in adaptive immune responses and innate immune responses.
Cell-mediated immunity is directed primarily at microbes that survive in phagocytes and microbes that infect non-phagocytic cells. It is most effective in removing virus-infected cells, but also participates in defending against fungi, protozoans, cancers, and intracellular bacteria. It also plays a major role in transplant rejection.
Source: Wikipedia
And Who says I study the dictionary?????
Jimmy B

Follow the "YELLOW BRICK ROAD!!!!!"
Cell-mediated immunity is an immune response that does not involve antibodies or complement but rather involves the activation of macrophages, natural killer cells (NK), antigen-specific cytotoxic T-lymphocytes (CTL), and the release of various cytokines in response to an antigen. Historically, the immune system was separated into two branches: humoral immunity, for which the protective function of immunization could be found in the humor (cell-free bodily fluid or serum) and cellular immunity, for which the protective function of immunization was associated with cells. CD4 cells or helper T cells provide protection against different pathogens.
Cellular immunity protects the body by:
1. activating antigen-specific cytotoxic T-lymphocytes(CTL)that are able to induce apoptosis in body cells displaying epitopes of foreign antigen on their surface, such as virus-infected cells, cells with intracellular bacteria, and cancer cells displaying tumor antigens;
2. activating macrophages and natural killer cells, enabling them to destroy intracellular pathogens; and
3. stimulating cells to secrete a variety of cytokines that influence the function of other cells involved in adaptive immune responses and innate immune responses.
Cell-mediated immunity is directed primarily at microbes that survive in phagocytes and microbes that infect non-phagocytic cells. It is most effective in removing virus-infected cells, but also participates in defending against fungi, protozoans, cancers, and intracellular bacteria. It also plays a major role in transplant rejection.
Source: Wikipedia
And Who says I study the dictionary?????
Jimmy B
Natural Killer T-cells!!!!! Melanoma Jim Breitfeller
Friday, February 20, 2009
We are all in it together!!!!!! And the Beat goes On! Melanoma Jim Breitfller
Sent this week:
Seeking access to some cutting edge Reports.
Thank for you consideration. This wasn’t really about me, it is about the army of Melanoma Patients out there that our desperately seeking new Therapies for melanoma. See, not all patients have the ability to seek the best Oncologist available as I did. They come from small, medium and large towns, and cites around the world. I myself had to travel five hours to get my treatment and or see my Oncologist, Dr. Kirkwood. With the knowledge in those reports/summaries, they could take that information to their Oncologists. See, I been well aware, that all small towns/cites have a don't have Melanoma Specialist. All they are seeking is the best available treatment including the cutting edge. I was hoping your company would help the Melanoma Cause.
See, I have a blog called Melanoma Missionary. I have seen first hand what inferior treatment can do. I have watched dozen of patients die because they went down the wrong path.
In 2009 over 64000 people in the US will be diagnosed with Melanoma and another 8400 will die as a result.
Is it time to set things aside and do what is right for the people?
Sincerly,
Jim Breitfeller
Seeking access to some cutting edge Reports.
Thank for you consideration. This wasn’t really about me, it is about the army of Melanoma Patients out there that our desperately seeking new Therapies for melanoma. See, not all patients have the ability to seek the best Oncologist available as I did. They come from small, medium and large towns, and cites around the world. I myself had to travel five hours to get my treatment and or see my Oncologist, Dr. Kirkwood. With the knowledge in those reports/summaries, they could take that information to their Oncologists. See, I been well aware, that all small towns/cites have a don't have Melanoma Specialist. All they are seeking is the best available treatment including the cutting edge. I was hoping your company would help the Melanoma Cause.
See, I have a blog called Melanoma Missionary. I have seen first hand what inferior treatment can do. I have watched dozen of patients die because they went down the wrong path.
In 2009 over 64000 people in the US will be diagnosed with Melanoma and another 8400 will die as a result.
Is it time to set things aside and do what is right for the people?
Sincerly,
Jim Breitfeller
Patty Luker, This for you!!! Melanoma..Jim Breitfeller
Patty Luker, This for you!!!
This so you don't go into the weekend empty handed

This is my rendition of an activated T-cell with anti CTLA-4 Blockage
T-cell activation requires not only stimulation of the T-cell receptor,but also a secondary costimulation if the CD28 receptors on the T-cell.
I guess my HOMEWORK is starting to payoff.
Have a great weekend Everybody!!!!!!
Take Care
Jimmy B
This so you don't go into the weekend empty handed

This is my rendition of an activated T-cell with anti CTLA-4 Blockage
T-cell activation requires not only stimulation of the T-cell receptor,but also a secondary costimulation if the CD28 receptors on the T-cell.
I guess my HOMEWORK is starting to payoff.
Have a great weekend Everybody!!!!!!
Take Care
Jimmy B
I believe I am Half way There!!!!!!!! Melanoma Jim Breitfeller
I believe I am Half way there with the article "Melanoma and the Magic Bullet (Monoclonal Antibodies)". I have been doing the final research and ask Dr. Weber if my theroy is too much hand waving or not. You are going to ask, who the hell is Dr. Weber. I am glad you asked.

"Dr. Weber is the director of the Donald A. Adam Comprehensive Melanoma Research Center at Moffitt Cancer Center, with the charge of bringing together basic scientists, clinical and translational investigators and prevention/epidemiology scientists in an integrated overall melanoma research effort that rapidly brings new drugs and ideas to the clinic. He works extensively with Drs. Vernon Sondak, James Mulé, Esteban Celis and Dmitri Gabrilovich, as well as others, to achieve these aims. Weber has an extensive history of conducting translational and investigator-initiated clinical trials. Dr. Weber is also a professor of Oncology and Medicine in the department of Interdisciplinary Oncology.
Dr. Weber received his doctorate in Molecular Cell Biology in 1979 from Rockefeller University. He received his medical degree from New York University Medical Center in 1980. He then completed an Internship and Residency in Medicine at the University of California. Dr. Weber also trained at the National Cancer Institute (1986-1990).
Dr. Weber’s clinical interests are in the immunotherapy of melanoma and other malignancies, with a focus on vaccines, adoptive immunotherapy, dendritic cell therapy and the use of immune modulating antibodies.
Dr. Weber’s research interests lie in the monitoring and characterization of T cell responses to vaccination in cancer patients, and in the establishment of in vitro models to facilitate the understanding of how immune modulating antibodies amplify T cell responses in patients. He is also interested in the mechanisms by which achieving autoimmunity induces regression of cancer
http://www.moffitt.org/Site.aspx?spid=F0B14BB585AB4205A5798A3E718234A0
Source:Dr. Weber
See, I want the information that I am going to provide is sound and realistic. I don't want to promise something that I can't deliver. I need to all my facts straight before releasing this information.
And that is why it is taking so long. I've been reseaching this for a long time and I want to present it right.
Take care
Jimmy B

"Dr. Weber is the director of the Donald A. Adam Comprehensive Melanoma Research Center at Moffitt Cancer Center, with the charge of bringing together basic scientists, clinical and translational investigators and prevention/epidemiology scientists in an integrated overall melanoma research effort that rapidly brings new drugs and ideas to the clinic. He works extensively with Drs. Vernon Sondak, James Mulé, Esteban Celis and Dmitri Gabrilovich, as well as others, to achieve these aims. Weber has an extensive history of conducting translational and investigator-initiated clinical trials. Dr. Weber is also a professor of Oncology and Medicine in the department of Interdisciplinary Oncology.
Dr. Weber received his doctorate in Molecular Cell Biology in 1979 from Rockefeller University. He received his medical degree from New York University Medical Center in 1980. He then completed an Internship and Residency in Medicine at the University of California. Dr. Weber also trained at the National Cancer Institute (1986-1990).
Dr. Weber’s clinical interests are in the immunotherapy of melanoma and other malignancies, with a focus on vaccines, adoptive immunotherapy, dendritic cell therapy and the use of immune modulating antibodies.
Dr. Weber’s research interests lie in the monitoring and characterization of T cell responses to vaccination in cancer patients, and in the establishment of in vitro models to facilitate the understanding of how immune modulating antibodies amplify T cell responses in patients. He is also interested in the mechanisms by which achieving autoimmunity induces regression of cancer
http://www.moffitt.org/Site.aspx?spid=F0B14BB585AB4205A5798A3E718234A0
Source:Dr. Weber
See, I want the information that I am going to provide is sound and realistic. I don't want to promise something that I can't deliver. I need to all my facts straight before releasing this information.
And that is why it is taking so long. I've been reseaching this for a long time and I want to present it right.
Take care
Jimmy B
CTLA-4 Therapy--Permission to used Diagrams..Melanoma Jim Breitfeller
Dr. Wolchok,
My name is Jim Breitfeller. I am a patient/survivor/researcher stage IV Melanoma under the care of Dr. Kirkwood. I completed a therapy that has prolonged my survival and would like to write a paper. Blog on the subject matter.
It is all about CTLA-4 Blockage therapy. Some of your diagrams and tables would be helpful in my presentation. I am asking for permission to use some of them. I will cite and give credit.
“The Mechanism of Anti-CTLA-4 Activity and the Negative Regulation of T-Cell Activation”
Thanks in advanced
Jim Breitfeller
Reply from Dr. Wolchok:
Dear Mr Breitfeller,
Thank you for your efforts. Please let me know which figures you would like to use. Some have been published and you may need permission from the publisher to re-use. I apologize if I cannot reply in an efficient fashion as patient care responsibilities and some urgent grant renewals are my top priority right now. I applaud your efforts.
Best wishes,
Jedd Wolchok
My name is Jim Breitfeller. I am a patient/survivor/researcher stage IV Melanoma under the care of Dr. Kirkwood. I completed a therapy that has prolonged my survival and would like to write a paper. Blog on the subject matter.
It is all about CTLA-4 Blockage therapy. Some of your diagrams and tables would be helpful in my presentation. I am asking for permission to use some of them. I will cite and give credit.
“The Mechanism of Anti-CTLA-4 Activity and the Negative Regulation of T-Cell Activation”
Thanks in advanced
Jim Breitfeller
Reply from Dr. Wolchok:
Dear Mr Breitfeller,
Thank you for your efforts. Please let me know which figures you would like to use. Some have been published and you may need permission from the publisher to re-use. I apologize if I cannot reply in an efficient fashion as patient care responsibilities and some urgent grant renewals are my top priority right now. I applaud your efforts.
Best wishes,
Jedd Wolchok
Thursday, February 19, 2009
Helpful hint of the day!!!!!!!! Melanoma.. Jim Breitfeller
Buy a calandar Book. You might want to get yourself a calandar book.The one with the two by two square for dates.
This way you can document what you did and who you saw on what date. A Chronological Medical history of your journey. I have been doing this for 4 years and it comes in handy when you need to refer back in time. When did I do that SCAN?
When did I do that SCAN?
And Yes, I do somethimes look like a NERD!!!!!
Jimmy B
This way you can document what you did and who you saw on what date. A Chronological Medical history of your journey. I have been doing this for 4 years and it comes in handy when you need to refer back in time.
 When did I do that SCAN?
When did I do that SCAN?And Yes, I do somethimes look like a NERD!!!!!
Jimmy B
Wednesday, February 18, 2009
Dr. Polly Matzinger Danger!! Danger!! Melanoma..Jim Breitfeller
Danger signals
"In a 1994 article entitled "Tolerance, Danger and the Extended Family", Matzinger went several steps further by laying out the idea that antigen-presenting cells respond to "danger signals" - most notably from cells undergoing injury, or stress or "bad cell death" (as opposed to apoptosis, controlled cell death). The alarm signals released by these cells let the immune system know that there is a problem requiring an immune response. She argued that T-cells and the immune response they orchestrate occurs not because of a neonatal definition of "self", as in the previous model, nor because of ancient definitions of pathogens, as in Janeway's argument, but due to a dynamic and constantly-updated response to danger as defined by cellular damage."
The Danger Model
The self-non-self model, the predominant model in immunology since the 1950s, began to encounter problems in the late 1980s when immunologists began to recognize that T-cells depend on other cells to pick up and then present the things to which they will respond — and that the T-cell response depends on whether the other cell (known as antigen-presenting cells) is sending activation signals to the T-cells.
In 1989, drawing on the ideas of Thomas Kuhn, Charles Janeway proposed that the old immunological paradigm had reached the limits of its usefulness--or, as he described it, the asymptote of the increase in knowledge which it had brought. Janeway argued that the innate immune system was the real gatekeeper of whether the immune system responded or did not respond. He also argued that the innate immune system used ancient pattern-recognition receptors to make these decisions - recognizing a pathogen by its unchanging characteristics.
Source:Wikipedia
Part of my Research
Jimmy B
"In a 1994 article entitled "Tolerance, Danger and the Extended Family", Matzinger went several steps further by laying out the idea that antigen-presenting cells respond to "danger signals" - most notably from cells undergoing injury, or stress or "bad cell death" (as opposed to apoptosis, controlled cell death). The alarm signals released by these cells let the immune system know that there is a problem requiring an immune response. She argued that T-cells and the immune response they orchestrate occurs not because of a neonatal definition of "self", as in the previous model, nor because of ancient definitions of pathogens, as in Janeway's argument, but due to a dynamic and constantly-updated response to danger as defined by cellular damage."
The Danger Model
The self-non-self model, the predominant model in immunology since the 1950s, began to encounter problems in the late 1980s when immunologists began to recognize that T-cells depend on other cells to pick up and then present the things to which they will respond — and that the T-cell response depends on whether the other cell (known as antigen-presenting cells) is sending activation signals to the T-cells.
In 1989, drawing on the ideas of Thomas Kuhn, Charles Janeway proposed that the old immunological paradigm had reached the limits of its usefulness--or, as he described it, the asymptote of the increase in knowledge which it had brought. Janeway argued that the innate immune system was the real gatekeeper of whether the immune system responded or did not respond. He also argued that the innate immune system used ancient pattern-recognition receptors to make these decisions - recognizing a pathogen by its unchanging characteristics.
Source:Wikipedia
Part of my Research
Jimmy B
Tuesday, February 17, 2009
Genomics: The convoluted promise of our generation ..Melanoma..Jim Breitfeller
Matthew B. May
Contributing Writer
Share this article Published: Sunday, February 15, 2009
Updated: Sunday, February 15, 2009
"There is little doubt that we live in the era of genomics, the biotechnological branch concerned with applying techniques to genetic mapping and DNA sequencing. While the struggles of the economic bailout and political wrangling of our discordant representatives might seem to define our generation, the greatest promises (and, no doubt, opportunities for entanglement) lie in the budding science of genomics.
What Tom Brokaw deemed “The Greatest Generation” for their contributions on a macro scale to the historical landscape ought soon be challenged by our toils to navigate the most uncharted and consequential frontier yet: the human genome.
A recent announcement by Complete Genomics, a Mountain View, Calif., company to provide entire genomic sequencings for $5,000 signifies the era of greater commercial and scientific utilization of genomic information. While companies such as Knome of Cambridge, Mass. provide both a genomic reading and interpreted analysis for $99,500, the era of available, interpretable and treatable consumer genomics remains something in our immediate future.
On one front, research into the genetic framework of disease has opened up expansive doors of insight into the molecular workings of our illnesses. Researchers at the University of Maryland School of Medicine have sequenced the genome of the common cold, a rhinovirus infection that costs the U.S. health care industry nearly $60 billion annually. The depth of knowledge uncovered about rhinoviruses, such as how they are organized into smaller groups and how strains possess the ability to exchange genetic information, provides a foundation for targeting the genetic intricacies that perpetuate the disease."
The rest can be found at Genomics: The convoluted promise of our generation
Take care
jimmy B
Contributing Writer
Share this article Published: Sunday, February 15, 2009
Updated: Sunday, February 15, 2009
"There is little doubt that we live in the era of genomics, the biotechnological branch concerned with applying techniques to genetic mapping and DNA sequencing. While the struggles of the economic bailout and political wrangling of our discordant representatives might seem to define our generation, the greatest promises (and, no doubt, opportunities for entanglement) lie in the budding science of genomics.
What Tom Brokaw deemed “The Greatest Generation” for their contributions on a macro scale to the historical landscape ought soon be challenged by our toils to navigate the most uncharted and consequential frontier yet: the human genome.
A recent announcement by Complete Genomics, a Mountain View, Calif., company to provide entire genomic sequencings for $5,000 signifies the era of greater commercial and scientific utilization of genomic information. While companies such as Knome of Cambridge, Mass. provide both a genomic reading and interpreted analysis for $99,500, the era of available, interpretable and treatable consumer genomics remains something in our immediate future.
On one front, research into the genetic framework of disease has opened up expansive doors of insight into the molecular workings of our illnesses. Researchers at the University of Maryland School of Medicine have sequenced the genome of the common cold, a rhinovirus infection that costs the U.S. health care industry nearly $60 billion annually. The depth of knowledge uncovered about rhinoviruses, such as how they are organized into smaller groups and how strains possess the ability to exchange genetic information, provides a foundation for targeting the genetic intricacies that perpetuate the disease."
The rest can be found at Genomics: The convoluted promise of our generation
Take care
jimmy B
Figure 1. T-cell Mobilized!!! Melanoma..Jim Breitfeller
Monday, February 16, 2009
If you are wondering where I am!!!!! Melanoma .. Jim Breitfeller
I am in the process of writing one of my technical post to date. It is still in the early stages but I want you to know that I AM STILL HERE.
THE TITLE:
"Melanoma and the “Magic Bullet” (Monoclonal Antibodies)
Introduction:
Paul Ehrlich - ( March, 14 1854 –August, 20 1915)
Dr. Ehrlich can be called the “Father of Modern Immunology”. He was a German scientist in the fields of hematology, immunology, and chemotherapy, and Nobel laureate. He is noted for his research in autoimmunity, calling it "horror autotoxicus". He coined the term "chemotherapy" and popularized the concept of a "magic bullet". He is credited with the first observation of the blood-brain barrier and the development of the first antibacterial drug in modern medicine.
The Magic Bullet concept was base on selective targeting a disease with a toxin/agent to kill off the disease without effecting the rest of the body. Using this concept in 1909, he and his student came up with a treatment for Syphilis.
One of his other works he is also famous for was called the “Side-Chain Theory” This proposed theory explaining the immune response in living cells.
The concept of a "magic bullet" was fully realized with the invention of monoclonal antibodies.
Today, we have a better understanding of our immune system but are still pushing back the
frontier in this area, as we try to decode the Mystery of Melanoma Cancer.
I just hope I can pull this off. I am going to have to make diagrams with the whole nine yards.
This will be my masterpiece to date.
It is not over until until it is over.
Take care
Jimmy B
THE TITLE:
"Melanoma and the “Magic Bullet” (Monoclonal Antibodies)
Introduction:
Paul Ehrlich - ( March, 14 1854 –August, 20 1915)
Dr. Ehrlich can be called the “Father of Modern Immunology”. He was a German scientist in the fields of hematology, immunology, and chemotherapy, and Nobel laureate. He is noted for his research in autoimmunity, calling it "horror autotoxicus". He coined the term "chemotherapy" and popularized the concept of a "magic bullet". He is credited with the first observation of the blood-brain barrier and the development of the first antibacterial drug in modern medicine.
The Magic Bullet concept was base on selective targeting a disease with a toxin/agent to kill off the disease without effecting the rest of the body. Using this concept in 1909, he and his student came up with a treatment for Syphilis.
One of his other works he is also famous for was called the “Side-Chain Theory” This proposed theory explaining the immune response in living cells.
The concept of a "magic bullet" was fully realized with the invention of monoclonal antibodies.
Today, we have a better understanding of our immune system but are still pushing back the
frontier in this area, as we try to decode the Mystery of Melanoma Cancer.
I just hope I can pull this off. I am going to have to make diagrams with the whole nine yards.
This will be my masterpiece to date.
It is not over until until it is over.
Take care
Jimmy B
Friday, February 13, 2009
A Conversation with Dr. Natalie Ahn ..Melanoma..Jim Breitfeller
Associate Professor at University of Colorado at Boulder
Ph.D.: University of California, Berkeley, 1985
Postdoctoral Fellow: University of Washington,
Awards:
Howard Hughes Medical Institute Investigator
Searle Scholar, 1993-1996
Merck Fellow, 1988-1991
Background:
“When Natalie Ahn was a postdoctoral student from 1988 to 1990 in the laboratory of Edwin Krebs at the University of Washington in Seattle, she was one of a group of scientists who discovered a signal transduction pathway (the mitogen activated protein or MAP kinase pathway) growth factors. Her approach was painstaking, requiring more than 10,000 assays. She also found an enzyme called MAP kinase kinase, which adds a phosphate to the enzyme MAP kinase to make it active.
Today, as a chemistry professor at the University of Colorado at Boulder, Ahn continues researching the subtleties of the MAP kinase and other signaling pathways, and their possible role in cancer. She also studies the fundamental chemical behavior of proteins.”
Source: Howard Hughs Medical Institute
Dr. Ahn, I as a Melanoma stage IV Patient/ Survivor applauded you and your colleagues on the research that you are doing for the Melanoma Cause.
Do you see some day that there will be genomic testing at the clinical level in the near future? Is the Molecular diagnostics keeping up with the research?
My type of Melanoma was the Nodular, which must be a mutation in the BRAF and or NRAS. Would doing the CTLA-4 therapy and then IL-2 correct those mutations? Or is it a temporarily response?
Any insight would be greatly appreciated.
To answer your last question first:
Mutations in B-Raf and N-Ras have been shown to cluster at specific nucleotides. This strongly suggests that there is a cellular mechanism which targets these sites in each gene. However, you are probably right, that anti-CTLA4 and IL2 are working by enhancing immune surveillance of your melanoma.
Therefore, I think it is unlikely that these therapies would reverse the mutations in cells which harbor them. On the other hand, it seems possible that immune therapy might somehow select for a subpopulation of melanoma cells within the heterogeneous tumors which lack mutations in B-Raf or N-Ras, so that after extended treatment, the cells with mutations die and cells without mutations expand. This could lead to an apparent switch in cell population within the tumors.
To answer your first two questions:
Yes, I believe that genomic testing will be feasible to predict disease susceptibility as well as treatment routes. This is already being used for some cancers. For example, markers such as estrogen receptor, progesterone receptor, or HER2 are already being used to predict responsiveness to different therapies for breast cancer. Such markers are less well developed for melanoma, but I think it is a matter of time and effort to discover and characterize them. One aim of my research is to develop new technologies for profiling proteins and chemical modifications of proteins, in hopes of identifying protein markers that can be used to diagnose melanomas and predict responsiveness to different therapies.
With respect to molecular diagnostics:
I think that there are many powerful technologies for molecular diagnostics -- such as gene chips which are used to survey transcripts. But other diagnostic technologies, e.g. for surveying proteins, are still immature. Also difficult is developing good laboratory medicine assays that can be performed with high accuracy and sensitivity. There are many blood markers that can be used to diagnose diseases, but a serious bottleneck is in the assays for quantifying these markers. So new technologies in laboratory medicine remains an important goal for the future.
I am also studying how B-Raf causes melanoma metastasis, and why targeted therapeutics which inhibit this pathway work in cells outside the body, but fail to work in patients. I believe that by studying mechanisms for resistance to these drugs, we might understand what else is needed in order to trigger melanomas to respond to these compounds. This would provide alternative therapies to melanoma patients, especially the many who fail to respond to current therapies.
Thank you for contacting me. I wish you the very best for your
treatment, and hope that your disease continues to remain stable for
many years to come.
Sincerely yours,
Natalie Ahn
Ph.D.: University of California, Berkeley, 1985
Postdoctoral Fellow: University of Washington,
Awards:
Howard Hughes Medical Institute Investigator
Searle Scholar, 1993-1996
Merck Fellow, 1988-1991
Background:
“When Natalie Ahn was a postdoctoral student from 1988 to 1990 in the laboratory of Edwin Krebs at the University of Washington in Seattle, she was one of a group of scientists who discovered a signal transduction pathway (the mitogen activated protein or MAP kinase pathway) growth factors. Her approach was painstaking, requiring more than 10,000 assays. She also found an enzyme called MAP kinase kinase, which adds a phosphate to the enzyme MAP kinase to make it active.
Today, as a chemistry professor at the University of Colorado at Boulder, Ahn continues researching the subtleties of the MAP kinase and other signaling pathways, and their possible role in cancer. She also studies the fundamental chemical behavior of proteins.”
Source: Howard Hughs Medical Institute
Dr. Ahn, I as a Melanoma stage IV Patient/ Survivor applauded you and your colleagues on the research that you are doing for the Melanoma Cause.
Do you see some day that there will be genomic testing at the clinical level in the near future? Is the Molecular diagnostics keeping up with the research?
My type of Melanoma was the Nodular, which must be a mutation in the BRAF and or NRAS. Would doing the CTLA-4 therapy and then IL-2 correct those mutations? Or is it a temporarily response?
Any insight would be greatly appreciated.
To answer your last question first:
Mutations in B-Raf and N-Ras have been shown to cluster at specific nucleotides. This strongly suggests that there is a cellular mechanism which targets these sites in each gene. However, you are probably right, that anti-CTLA4 and IL2 are working by enhancing immune surveillance of your melanoma.
Therefore, I think it is unlikely that these therapies would reverse the mutations in cells which harbor them. On the other hand, it seems possible that immune therapy might somehow select for a subpopulation of melanoma cells within the heterogeneous tumors which lack mutations in B-Raf or N-Ras, so that after extended treatment, the cells with mutations die and cells without mutations expand. This could lead to an apparent switch in cell population within the tumors.
To answer your first two questions:
Yes, I believe that genomic testing will be feasible to predict disease susceptibility as well as treatment routes. This is already being used for some cancers. For example, markers such as estrogen receptor, progesterone receptor, or HER2 are already being used to predict responsiveness to different therapies for breast cancer. Such markers are less well developed for melanoma, but I think it is a matter of time and effort to discover and characterize them. One aim of my research is to develop new technologies for profiling proteins and chemical modifications of proteins, in hopes of identifying protein markers that can be used to diagnose melanomas and predict responsiveness to different therapies.
With respect to molecular diagnostics:
I think that there are many powerful technologies for molecular diagnostics -- such as gene chips which are used to survey transcripts. But other diagnostic technologies, e.g. for surveying proteins, are still immature. Also difficult is developing good laboratory medicine assays that can be performed with high accuracy and sensitivity. There are many blood markers that can be used to diagnose diseases, but a serious bottleneck is in the assays for quantifying these markers. So new technologies in laboratory medicine remains an important goal for the future.
I am also studying how B-Raf causes melanoma metastasis, and why targeted therapeutics which inhibit this pathway work in cells outside the body, but fail to work in patients. I believe that by studying mechanisms for resistance to these drugs, we might understand what else is needed in order to trigger melanomas to respond to these compounds. This would provide alternative therapies to melanoma patients, especially the many who fail to respond to current therapies.
Thank you for contacting me. I wish you the very best for your
treatment, and hope that your disease continues to remain stable for
many years to come.
Sincerely yours,
Natalie Ahn
Wednesday, February 11, 2009
A Stage IV Malignant Melanoma Drug That Increases Overall Survival Would Earn a Higher Patient Share in the U.S. Than in Europe..Jim Breitfeller
Ipilimumab Will Earn Decision Resources' Clinical Gold Standard for stage IV Malignant melanoma in 2012, According to a New Report from Decision Resources
Ipilimumab is a fully human antibody that binds to CTLA-4 (cytotoxic T lymphocyte-associated antigen 4), a molecule on T-cells that plays a critical role in regulating natural immune responses. The absence or presence of CTLA- 4 can augment or suppress the immune system's T-cell response in fighting disease. Ipilimumab is designed to block the activity of CTLA-4, thereby sustaining an active immune response in its attack on cancer cells.
WALTHAM, Mass., Jan. 19 /PRNewswire/ -- Decision Resources, one of the world's leading research and advisory firms for pharmaceutical and healthcare issues, finds that a drug for treating stage IV malignant melanoma that can increase median overall survival when compared with standard of care dacarbazine (Bedford Laboratories' DTIC-Dome, generics) would earn a higher patient share in the U.S. (60 percent) than in Europe (40 percent), according to surveyed U.S. and European oncologists.
The new report entitled Malignant melanoma (Stage IV): Emerging Therapies Must Increase Overall Survival over Dacarbazine to Attain High Patient Share finds that clinical data and the opinions of interviewed thought leaders indicate that Bristol-Myers Squibb/Medarex's ipilimumab has advantages over dacarbazine in the attribute of median overall survival. Following its approval in 2010 for the indication, ipilimumab will earn Decision Resources' proprietary clinical gold standard for stage IV malignant melanoma from 2012 to 2017.
"Ipilimumab has competitive advantages in efficacy and has been shown, in combination with dacarbazine, to almost double median overall survival when compared to Schering-Plough's Temodar, our clinical gold standard in 2008 for stage IV malignant melanoma," said Decision Resources Analyst Karen Pomeranz, Ph.D. "According to oncologists we surveyed, overall survival is the highest-weighted end point for stage IV malignant melanoma, which further stresses the significance of ipilimumab's achievement in increasing overall survival."
http://www.bio-medicine.org/medicine-news-1/A-Stage-IV-Malignant-Melanoma-Drug-That-Increases-Overall-Survival-Would-Earn-a-Higher-Patient-Share-in-the-U-S--Than-in-Europe-34223-1/
A Stage IV Malignant Melanoma Drug That Increases Overall Survival Would Earn a Higher Patient Share in the U.S. Than in Europe
Like I said CTLA-4 therapy with another additive therapy
Jimmy B
Ipilimumab is a fully human antibody that binds to CTLA-4 (cytotoxic T lymphocyte-associated antigen 4), a molecule on T-cells that plays a critical role in regulating natural immune responses. The absence or presence of CTLA- 4 can augment or suppress the immune system's T-cell response in fighting disease. Ipilimumab is designed to block the activity of CTLA-4, thereby sustaining an active immune response in its attack on cancer cells.
WALTHAM, Mass., Jan. 19 /PRNewswire/ -- Decision Resources, one of the world's leading research and advisory firms for pharmaceutical and healthcare issues, finds that a drug for treating stage IV malignant melanoma that can increase median overall survival when compared with standard of care dacarbazine (Bedford Laboratories' DTIC-Dome, generics) would earn a higher patient share in the U.S. (60 percent) than in Europe (40 percent), according to surveyed U.S. and European oncologists.
The new report entitled Malignant melanoma (Stage IV): Emerging Therapies Must Increase Overall Survival over Dacarbazine to Attain High Patient Share finds that clinical data and the opinions of interviewed thought leaders indicate that Bristol-Myers Squibb/Medarex's ipilimumab has advantages over dacarbazine in the attribute of median overall survival. Following its approval in 2010 for the indication, ipilimumab will earn Decision Resources' proprietary clinical gold standard for stage IV malignant melanoma from 2012 to 2017.
"Ipilimumab has competitive advantages in efficacy and has been shown, in combination with dacarbazine, to almost double median overall survival when compared to Schering-Plough's Temodar, our clinical gold standard in 2008 for stage IV malignant melanoma," said Decision Resources Analyst Karen Pomeranz, Ph.D. "According to oncologists we surveyed, overall survival is the highest-weighted end point for stage IV malignant melanoma, which further stresses the significance of ipilimumab's achievement in increasing overall survival."
http://www.bio-medicine.org/medicine-news-1/A-Stage-IV-Malignant-Melanoma-Drug-That-Increases-Overall-Survival-Would-Earn-a-Higher-Patient-Share-in-the-U-S--Than-in-Europe-34223-1/
A Stage IV Malignant Melanoma Drug That Increases Overall Survival Would Earn a Higher Patient Share in the U.S. Than in Europe
Like I said CTLA-4 therapy with another additive therapy
Jimmy B
Monday, February 9, 2009
Micro RNA Plays A Key Role In Melanoma Metastasis..Jim Breitfeller
Adapted from materials provided by NYU Langone Medical Center / New York University School of Medicine.
ScienceDaily (Feb. 9, 2009) — Scientists have long wondered how melanoma cells travel from primary tumors on the surface of the skin to the brain, liver and lungs, where they become more aggressive, resistant to therapy, and deadly. Now, scientists from NYU Langone Medical Center have identified the possible culprit—a short strand of RNA called microRNA (miRNA) that is over-expressed in metastatic melanoma cell lines and tissues
Source:
RNA Plays A Key Role In Melanoma Metastasis
The new findings, published February 10, 2009 in the Proceedings of the National Academy of Sciences (PNAS), suggest that miRNA silencing to counteract or attack this mechanism may be an effective therapeutic strategy for metastatic melanoma, according to Eva Hernando, Ph.D., assistant professor in the Department of Pathology at NYU School of Medicine, and the lead author of the study. Dr. Hernando is also a member of the NYU Cancer Institute at NYU Langone Medical Center.
The highly aggressive character of melanoma, says Dr. Hernando, makes it an excellent model to probe the mechanisms underlying metastasis, the process by which cancer cells travel from the primary tumor to distant sites in the body. Though other researchers have found that altered miRNAs contribute to breast cancer metastasis, this is the first study to examine the role of miRNA in metastatic melanoma.
"Melanoma becomes deadly after the cells leave the primary tumor through the blood and metastasize in other organs where they are resistant to therapy," says Dr. Hernando, who notes that the average survival for patients after melanoma metastasis occurs is only nine months. "Normal cells are unable to travel and survive in alien locations, so we are very interested in understanding the invasive, adaptive, and resistant traits of the very aggressive melanoma cell." miRNAs are short pieces of RNA that block the expression of proteins that are encoded by messenger RNAs. They serve as regulators of protein expression, acting like the volume control on a radio. In recent years, miRNAs have been linked to the over- or under-expression of a variety of genes linked to cancer and other diseases.
Dr. Hernando's lab found a miRNA is over-expressed in metastatic melanoma cell lines and tissues. The lab found that the elevated expression of miRNA 182 turns it into an oncogene (a gene involved in cancer tumor initiation or progression), by increasing the invasive capacity of melanoma cells in vitro and stimulating the cell's metastatic potential in a mouse model.
In addition, the NYU scientists found that miRNA 182 also represses the expression of two tumor suppressors called FOXO3 and MITF, which normally prevent cells from becoming malignant. By repressing the suppressors, miRNA 182 permits melanoma cells to migrate and survive independently, two properties necessary for metastasis.
MiRNA 182 also belongs to a cluster located in a genomic region, chromosome 7q, that is frequently amplified in melanoma and contains two other oncogenes; BRAF and C-MET. The study found a correlation between genomic amplification and miRNA over expression, though it is unclear whether other molecular mechanisms play a role in this effect, according to Dr. Hernando.
Finally, the scientists observed that in a significant fraction of metastatic melanomas, high miRNA 182 levels correlate with low levels of FOXO3 and MITF, supporting the relevance of this mechanism in human melanoma.
The study suggests that miRNA 182 is a novel therapeutic target. When it is inhibited, it impairs the invasive potential of melanoma cells and induces cell death. In theory, the administration of anti-miRNA 182 could block the growth or expansion of the primary melanoma tumor. Several academic laboratories and pharmaceutical companies are working to improve the delivery of anti-miRNAs by using chemical modification and nano particles to increase their stability, specificity, and ability to reach tumors in sufficient doses with low toxicity.
The NYU Cancer Institute is currently studying whether anti-miRNA will work on miRNA 182 to inhibit the growth or spread of primary melanoma in mice. Dr. Hernando says that even if the anti-miRNA cannot do this on its own, it might work in combination with conventional chemotherapy or novel targeted therapies.
This study is the result of an extensive collaboration between members of NYU's Interdisciplinary Melanoma Cooperative Group, led by Iman Osman, M.D. , one of the study's co-authors, which has a large biospecimen bank comprising human tissue, blood and patient clinico-pathological information.
"The existence of this bank permits us to validate our laboratory findings using human tissue," says Dr. Hernando. "In this study, we began looking at cell lines and then at melanoma tissue. Now that the mechanism has been proven using cell lines and mice, the next step will be to perform in-vitro studies with cell lines to assess the effect of anti-miRNA on cell death in both normal and melanoma cells. Once that study is completed, we can use this model for studies in mice to block the growth of the primary melanoma tumor or the metastasis by using anti-miRNA. All these steps will determine if this approach could be eventually applied to humans."
Jimmy B
ScienceDaily (Feb. 9, 2009) — Scientists have long wondered how melanoma cells travel from primary tumors on the surface of the skin to the brain, liver and lungs, where they become more aggressive, resistant to therapy, and deadly. Now, scientists from NYU Langone Medical Center have identified the possible culprit—a short strand of RNA called microRNA (miRNA) that is over-expressed in metastatic melanoma cell lines and tissues
Source:
RNA Plays A Key Role In Melanoma Metastasis
The new findings, published February 10, 2009 in the Proceedings of the National Academy of Sciences (PNAS), suggest that miRNA silencing to counteract or attack this mechanism may be an effective therapeutic strategy for metastatic melanoma, according to Eva Hernando, Ph.D., assistant professor in the Department of Pathology at NYU School of Medicine, and the lead author of the study. Dr. Hernando is also a member of the NYU Cancer Institute at NYU Langone Medical Center.
The highly aggressive character of melanoma, says Dr. Hernando, makes it an excellent model to probe the mechanisms underlying metastasis, the process by which cancer cells travel from the primary tumor to distant sites in the body. Though other researchers have found that altered miRNAs contribute to breast cancer metastasis, this is the first study to examine the role of miRNA in metastatic melanoma.
"Melanoma becomes deadly after the cells leave the primary tumor through the blood and metastasize in other organs where they are resistant to therapy," says Dr. Hernando, who notes that the average survival for patients after melanoma metastasis occurs is only nine months. "Normal cells are unable to travel and survive in alien locations, so we are very interested in understanding the invasive, adaptive, and resistant traits of the very aggressive melanoma cell." miRNAs are short pieces of RNA that block the expression of proteins that are encoded by messenger RNAs. They serve as regulators of protein expression, acting like the volume control on a radio. In recent years, miRNAs have been linked to the over- or under-expression of a variety of genes linked to cancer and other diseases.
Dr. Hernando's lab found a miRNA is over-expressed in metastatic melanoma cell lines and tissues. The lab found that the elevated expression of miRNA 182 turns it into an oncogene (a gene involved in cancer tumor initiation or progression), by increasing the invasive capacity of melanoma cells in vitro and stimulating the cell's metastatic potential in a mouse model.
In addition, the NYU scientists found that miRNA 182 also represses the expression of two tumor suppressors called FOXO3 and MITF, which normally prevent cells from becoming malignant. By repressing the suppressors, miRNA 182 permits melanoma cells to migrate and survive independently, two properties necessary for metastasis.
MiRNA 182 also belongs to a cluster located in a genomic region, chromosome 7q, that is frequently amplified in melanoma and contains two other oncogenes; BRAF and C-MET. The study found a correlation between genomic amplification and miRNA over expression, though it is unclear whether other molecular mechanisms play a role in this effect, according to Dr. Hernando.
Finally, the scientists observed that in a significant fraction of metastatic melanomas, high miRNA 182 levels correlate with low levels of FOXO3 and MITF, supporting the relevance of this mechanism in human melanoma.
The study suggests that miRNA 182 is a novel therapeutic target. When it is inhibited, it impairs the invasive potential of melanoma cells and induces cell death. In theory, the administration of anti-miRNA 182 could block the growth or expansion of the primary melanoma tumor. Several academic laboratories and pharmaceutical companies are working to improve the delivery of anti-miRNAs by using chemical modification and nano particles to increase their stability, specificity, and ability to reach tumors in sufficient doses with low toxicity.
The NYU Cancer Institute is currently studying whether anti-miRNA will work on miRNA 182 to inhibit the growth or spread of primary melanoma in mice. Dr. Hernando says that even if the anti-miRNA cannot do this on its own, it might work in combination with conventional chemotherapy or novel targeted therapies.
This study is the result of an extensive collaboration between members of NYU's Interdisciplinary Melanoma Cooperative Group, led by Iman Osman, M.D. , one of the study's co-authors, which has a large biospecimen bank comprising human tissue, blood and patient clinico-pathological information.
"The existence of this bank permits us to validate our laboratory findings using human tissue," says Dr. Hernando. "In this study, we began looking at cell lines and then at melanoma tissue. Now that the mechanism has been proven using cell lines and mice, the next step will be to perform in-vitro studies with cell lines to assess the effect of anti-miRNA on cell death in both normal and melanoma cells. Once that study is completed, we can use this model for studies in mice to block the growth of the primary melanoma tumor or the metastasis by using anti-miRNA. All these steps will determine if this approach could be eventually applied to humans."
Jimmy B
Did the clinical trials on CTLA-4 Therapy Sell us short and we missed the boat??..Melanoma.. Jim Breitfeller
My experience with CTLA-4 Blockage Therapy:
On 9-13-2006 I received my first and only dose of CTLA-4.
9-19-2006 – “The New CTLA-4 trial, has sent me for a loop. I wake up exhausted. I have to push myself to get out of bed. Along with the fatigue, my muscles ache like they have lactic acid in them. Well, no pain no gain.”
9/27/06—“Saw the Physician Assistant, Melissa. It appears that the CTLA-4 has stimulated my immune system. In the pass week, I noticed that there was redness around the area where my tumors are located. Also it is becoming quite tender in that area. This is Great news!!!!! It appears that the treatment my have kick started my immune system. The only way we will know for sure is another CT scan. That is not scheduled until November 29th .
Oct 10, 2006—“I still have fatigue but I am managing it. What really bothers me is my right arm where they removed the lymph nodes. I can't get the swelling down which in turn puts pressure on my nerves. I have to baby it to try to bring down the swelling. Some days it feels like it is caught in a vise. I am going ask if they can drain the excess fluid if possible.”
Oct 12, 2006—“A couple of days ago, Dee noticed two new growths on my back. I was hoping for the best. Anyway, we got confirmation from the Hillman Center that it is 2 new tumors growing. This really stinks. I think it is time to take out the “Weed be Gone”. This is not what I was hoping to hear.” The therapy with CTLA-4 was terminated”.
“So, it is on to the next trial. I am not sure what is going to be, but they mention Interleukin -2.”
“Improved understanding of the functions of CTLA-4 led to the hypothesis that blocking its engagement could result in unopposed CD28 activation of T-cells coupled with suppression or depletion of regulatory cells. In essence, blockade of CTLA-4 leads to "taking the brakes off" the immune system.”1
Antitumor activity has been observed in melanoma after treatment with anti-CTLA-4 antibodies, as well as the potential for autoimmune-related toxicities. The Anti-CTLA-4 antibodies would bind to the B7 receptor. This in turn would keep the T-Cells activated for a prolonged time.
“It has been seen that CTLA-4 antibody blockade alone has had minimal effects in other mouse tumors, however, including the poorly immunogenic B16 melanoma2 and SM1 breast cancer.3 However, the combination of CTLA-4 blockade and vaccination with GVAX (irradiated tumor cells engineered to secrete cytokine granulocyte-macrophage colony-stimulating factor [GM-CSF]) resulted in significant tumor regression in B16 melanoma.2”
The immune system is a powerful weapon, and the body does not use it lightly. Cells in this system must all be activated before they can begin proliferating and carrying out their instructions. For T-cells, two steps are required for activation. This safeguard can be likened to the two keys needed to open safe deposit boxes, in which the box holder has a key unique to that box, and the bank teller has a key that fits all boxes, but both keys are needed to open any particular box.4
This first interaction involves the CD4 or CD8 proteins which form a complex with the CD3 protein to bind to the MHC molecule of the (APC). Antigen-presenting cell This is also called "Signal 1" and its main purpose is T cell activation. IL-2 aids in the “Cell to Cell” communication.
However, this is insufficient for producing a T cell response by itself. In fact, lack of further stimulatory signals sends the T cell into anergy. Anergy is a term in immunobiology that describes a lack of reaction by the body's defense mechanisms to foreign substances.
The Second costimulatory signal necessary to continue the immune response can come from B7-CD28 and CD40-CD40L interactions. The primary role of the B7 proteins is to give a second signal to the T cell. The B7 protein/receptor is present on the Antigen-presenting cell and is able to interact with the CD28 receptor on the T cell surface; this is also known as "Signal 2". There are other activation signals which play a role in immune responses.
On these T cells there is are family receptors whose job is downregulate the T cell activation so the immune system maintains metabolic equilibrium so the immune system doesn’t start an autoimmune response and cause it to attack itself. One of these receptors is Cytotoxic T lymphocyte-associated antigen (CTLA4). So Using the CTLA-4 blockade, the two keys are there to produce the wanted immune response which is to attack the foreign molecule (The Tumor)
Tremelimumab and ipilimumab, two fully human Monoclonal Antibodies (mAbs) that block CTLA-4, have progressed into clinical trials and demonstrated antitumor activity in cancer patients. Most clinical investigations have been performed in patients with advanced melanoma .
In looking at Phase 1 and Phase 2 Clinical trials:
Table 1. Phase I and II Clinical Trials of CTLA-4 Blockade in Metastatic Melanoma5
In the Living Medical Textbook “Oncology” Chapter 4 and looking at the Efficacy Data, I notice that Dr. Maker had the best (CR) complete response 8%.
“Tumor Regression and Autoimmunity in Patients Treated With Cytotoxic T Lymphocyte–Associated Antigen 4 Blockade and Interleukin 2: A Phase I/II Study”
The way I derived the percentage was to take the number of complete responses and divide it by the number of patients in the trial, times 100. I also noticed that most of the complete responses were with a combination / additional therapy. This is SCREAMING OUT that this agent needs to be done in combination with other agents.
When Pfizer did there phase three trial against dacarbizine, the trial was done as a single agent. “Pfizer's randomized phase III of tremelimumab versus the fool's gold standard dacarabzine/DTIC was stopped due to futility.”6
I believe that CTLA4 blockade has clinically significant activity in melanoma and that we need to come back and address how to use this agent in combination with other agents.
I know that Dr. Rosenberg has tried with IL-2 and saw no additive improvement. I believe the trial was flawed in that the dosing was given at the same time.
“We have just not learned how to use these drugs to their maximum effect. Strategies that focus on complementary combinations intended to increase their immunostimulatory effect seem to be the right path. I just hope that we will not abandon CTLA4 blockade based merely on results of protocols that were not fair assessments of their possible activity”6
I am an example of the right Protocol:
“On September 6th, I had another CT scan and MRI for the next trial which was Anti-CTLA-4 Blockage . On September 13 I had my first infusion. On October 8, 2006 My wife noticed two new growths on my back. It was confirmed on October 11th it was new tumors.
Dr. John Kirkwood has decided that IL 2 Interleukin 2 would be next course of action.
I have completed another round of tests (CT scan, Pulmonary Function, and a Nuclear Stress Test). The CT scans shows 40+ nodules in my lungs ranging from 15mm to less than 5mm.
I am slated to be High dosage IL-2 on November 1st 2006.
I am presently washed out an IL-2 clinical trial that started in November 1, 2006. On the fourth cycle I had a heart attack and the doctors determined to abort the IL-2 on February 2, 2007.
On August 23 2007 there was no change to the tumors in my back or lungs but also no growth.
In October 24 2007 I got the word that the tumors and the lung nodules were shrinking.
In April 14, 2008, the 40 + nodules in my lungs decrease to 2.
In July 2008, the nodules in my lungs were undetectable and the ones on my back were all but one gone. Presently, CT and MRI
In November 2008 show no signs cancerous activity.
I did one cycle of CTLA-4, during that time I notice that there was redness around the tumor area and was soar. At the time we (the physician assistant) thought it was an infection. In retrospect, it was the activation on my T cells. Two new tumors appeared and it was decided to go on to interleukin-2 (High dose). Well , I believed that the IL-2 started the cell to cell communication, which was the costimulant to the T cell and there my immune system began the assault on the foreign invaders (the tumors).
I have seen this with another patient but did the IL-2 first with no success. But then went on to the CTLA-4 therapy and the tumors shrank.
I believe we are on to some thing big!!!!!!!!!! We must Investigate this combination therapy.
Jimmy B
References
1. Living Medical Textbook “Oncology” From Projects In Knowledge Contributing Writers: F. Stephen Hodi, MD and Lauren Cerruto http://www.livingmedicaltextbook.org/Activity/index.cfm?showfile=b&jn=1843&sj=1843.01&sc=1843.01.4
2. Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355-366.
3. Hurwitz AA, Yu T F-Y, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimentral mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067-10071.
4. Sompayrac L. How the Immune System Works. 3rd ed. Malden, MA: Blackwell Publishing; 2008
5. Table 1. Phase I and II Clinical Trials of CTLA-4 Blockade in Metastatic Melanoma5 http://www.livingmedicaltextbook.org/Activity/index.cfm?showfile=b&jn=1843&sj=1843.04&sc=1843.04.2
6. http://www.glgroup.com/News/CTLA4-Blockade-in-Melanoma--Where-Next--23639.html
Jimmy B
On 9-13-2006 I received my first and only dose of CTLA-4.
9-19-2006 – “The New CTLA-4 trial, has sent me for a loop. I wake up exhausted. I have to push myself to get out of bed. Along with the fatigue, my muscles ache like they have lactic acid in them. Well, no pain no gain.”
9/27/06—“Saw the Physician Assistant, Melissa. It appears that the CTLA-4 has stimulated my immune system. In the pass week, I noticed that there was redness around the area where my tumors are located. Also it is becoming quite tender in that area. This is Great news!!!!! It appears that the treatment my have kick started my immune system. The only way we will know for sure is another CT scan. That is not scheduled until November 29th .
Oct 10, 2006—“I still have fatigue but I am managing it. What really bothers me is my right arm where they removed the lymph nodes. I can't get the swelling down which in turn puts pressure on my nerves. I have to baby it to try to bring down the swelling. Some days it feels like it is caught in a vise. I am going ask if they can drain the excess fluid if possible.”
Oct 12, 2006—“A couple of days ago, Dee noticed two new growths on my back. I was hoping for the best. Anyway, we got confirmation from the Hillman Center that it is 2 new tumors growing. This really stinks. I think it is time to take out the “Weed be Gone”. This is not what I was hoping to hear.” The therapy with CTLA-4 was terminated”.
“So, it is on to the next trial. I am not sure what is going to be, but they mention Interleukin -2.”
“Improved understanding of the functions of CTLA-4 led to the hypothesis that blocking its engagement could result in unopposed CD28 activation of T-cells coupled with suppression or depletion of regulatory cells. In essence, blockade of CTLA-4 leads to "taking the brakes off" the immune system.”1
Antitumor activity has been observed in melanoma after treatment with anti-CTLA-4 antibodies, as well as the potential for autoimmune-related toxicities. The Anti-CTLA-4 antibodies would bind to the B7 receptor. This in turn would keep the T-Cells activated for a prolonged time.
“It has been seen that CTLA-4 antibody blockade alone has had minimal effects in other mouse tumors, however, including the poorly immunogenic B16 melanoma2 and SM1 breast cancer.3 However, the combination of CTLA-4 blockade and vaccination with GVAX (irradiated tumor cells engineered to secrete cytokine granulocyte-macrophage colony-stimulating factor [GM-CSF]) resulted in significant tumor regression in B16 melanoma.2”
The immune system is a powerful weapon, and the body does not use it lightly. Cells in this system must all be activated before they can begin proliferating and carrying out their instructions. For T-cells, two steps are required for activation. This safeguard can be likened to the two keys needed to open safe deposit boxes, in which the box holder has a key unique to that box, and the bank teller has a key that fits all boxes, but both keys are needed to open any particular box.4
This first interaction involves the CD4 or CD8 proteins which form a complex with the CD3 protein to bind to the MHC molecule of the (APC). Antigen-presenting cell This is also called "Signal 1" and its main purpose is T cell activation. IL-2 aids in the “Cell to Cell” communication.
However, this is insufficient for producing a T cell response by itself. In fact, lack of further stimulatory signals sends the T cell into anergy. Anergy is a term in immunobiology that describes a lack of reaction by the body's defense mechanisms to foreign substances.
The Second costimulatory signal necessary to continue the immune response can come from B7-CD28 and CD40-CD40L interactions. The primary role of the B7 proteins is to give a second signal to the T cell. The B7 protein/receptor is present on the Antigen-presenting cell and is able to interact with the CD28 receptor on the T cell surface; this is also known as "Signal 2". There are other activation signals which play a role in immune responses.
On these T cells there is are family receptors whose job is downregulate the T cell activation so the immune system maintains metabolic equilibrium so the immune system doesn’t start an autoimmune response and cause it to attack itself. One of these receptors is Cytotoxic T lymphocyte-associated antigen (CTLA4). So Using the CTLA-4 blockade, the two keys are there to produce the wanted immune response which is to attack the foreign molecule (The Tumor)
Tremelimumab and ipilimumab, two fully human Monoclonal Antibodies (mAbs) that block CTLA-4, have progressed into clinical trials and demonstrated antitumor activity in cancer patients. Most clinical investigations have been performed in patients with advanced melanoma .
In looking at Phase 1 and Phase 2 Clinical trials:
Table 1. Phase I and II Clinical Trials of CTLA-4 Blockade in Metastatic Melanoma5
In the Living Medical Textbook “Oncology” Chapter 4 and looking at the Efficacy Data, I notice that Dr. Maker had the best (CR) complete response 8%.
“Tumor Regression and Autoimmunity in Patients Treated With Cytotoxic T Lymphocyte–Associated Antigen 4 Blockade and Interleukin 2: A Phase I/II Study”
The way I derived the percentage was to take the number of complete responses and divide it by the number of patients in the trial, times 100. I also noticed that most of the complete responses were with a combination / additional therapy. This is SCREAMING OUT that this agent needs to be done in combination with other agents.
When Pfizer did there phase three trial against dacarbizine, the trial was done as a single agent. “Pfizer's randomized phase III of tremelimumab versus the fool's gold standard dacarabzine/DTIC was stopped due to futility.”6
I believe that CTLA4 blockade has clinically significant activity in melanoma and that we need to come back and address how to use this agent in combination with other agents.
I know that Dr. Rosenberg has tried with IL-2 and saw no additive improvement. I believe the trial was flawed in that the dosing was given at the same time.
“We have just not learned how to use these drugs to their maximum effect. Strategies that focus on complementary combinations intended to increase their immunostimulatory effect seem to be the right path. I just hope that we will not abandon CTLA4 blockade based merely on results of protocols that were not fair assessments of their possible activity”6
I am an example of the right Protocol:
“On September 6th, I had another CT scan and MRI for the next trial which was Anti-CTLA-4 Blockage . On September 13 I had my first infusion. On October 8, 2006 My wife noticed two new growths on my back. It was confirmed on October 11th it was new tumors.
Dr. John Kirkwood has decided that IL 2 Interleukin 2 would be next course of action.
I have completed another round of tests (CT scan, Pulmonary Function, and a Nuclear Stress Test). The CT scans shows 40+ nodules in my lungs ranging from 15mm to less than 5mm.
I am slated to be High dosage IL-2 on November 1st 2006.
I am presently washed out an IL-2 clinical trial that started in November 1, 2006. On the fourth cycle I had a heart attack and the doctors determined to abort the IL-2 on February 2, 2007.
On August 23 2007 there was no change to the tumors in my back or lungs but also no growth.
In October 24 2007 I got the word that the tumors and the lung nodules were shrinking.
In April 14, 2008, the 40 + nodules in my lungs decrease to 2.
In July 2008, the nodules in my lungs were undetectable and the ones on my back were all but one gone. Presently, CT and MRI
In November 2008 show no signs cancerous activity.
I did one cycle of CTLA-4, during that time I notice that there was redness around the tumor area and was soar. At the time we (the physician assistant) thought it was an infection. In retrospect, it was the activation on my T cells. Two new tumors appeared and it was decided to go on to interleukin-2 (High dose). Well , I believed that the IL-2 started the cell to cell communication, which was the costimulant to the T cell and there my immune system began the assault on the foreign invaders (the tumors).
I have seen this with another patient but did the IL-2 first with no success. But then went on to the CTLA-4 therapy and the tumors shrank.
I believe we are on to some thing big!!!!!!!!!! We must Investigate this combination therapy.
Jimmy B
References
1. Living Medical Textbook “Oncology” From Projects In Knowledge Contributing Writers: F. Stephen Hodi, MD and Lauren Cerruto http://www.livingmedicaltextbook.org/Activity/index.cfm?showfile=b&jn=1843&sj=1843.01&sc=1843.01.4
2. Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355-366.
3. Hurwitz AA, Yu T F-Y, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimentral mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067-10071.
4. Sompayrac L. How the Immune System Works. 3rd ed. Malden, MA: Blackwell Publishing; 2008
5. Table 1. Phase I and II Clinical Trials of CTLA-4 Blockade in Metastatic Melanoma5 http://www.livingmedicaltextbook.org/Activity/index.cfm?showfile=b&jn=1843&sj=1843.04&sc=1843.04.2
6. http://www.glgroup.com/News/CTLA4-Blockade-in-Melanoma--Where-Next--23639.html
Jimmy B
Labels:
CTLA-4,
cytokine,
dr. kirkwood,
Melanoma,
Rosenberg
Saturday, February 7, 2009
Clinical Care Options!!! Melanoma Jim Breitfeller
I Know it is the weekend, but Cancer doesn't take it off and neither do I.
In my Research I came upon a website that may Help you understand Melanoma a bit better. It is called Clinical Care Options.
http://www.clinicaloptions.com/
Clinical Care Options
You must signup to be able to view the modules. It is free and it is well worth investigating. It is an Educational site with modules that well Known Oncologists have put together for the Oncology Field.
After you Signup,go to the Clinical Care Options "Oncology"
Type in Melanoma in the search field at the top of the page. You will see pages of modules to choose from.
Take your Pick!!!!
This is a great source of information.
Welcome!
Thank you for registering with the Clinical Care Options web site.
Your Current User Name is: Jimmy_b
You can change either of these by visiting http://clinicaloptions.com/ and going to "My Profile" from the web site header.
Be sure to check out:
Expert Analysis programs from major medical meetings (CME certified)
Downloadable PowerPoint Slides to use in your talks
Journal Options, a summary of clinically relevant new publications (CME certified)
We are always interested in feedback from our members.
Please email memberservices@clinicaloptions.com with any comments or questions
Jimmy B
In my Research I came upon a website that may Help you understand Melanoma a bit better. It is called Clinical Care Options.
http://www.clinicaloptions.com/
Clinical Care Options
You must signup to be able to view the modules. It is free and it is well worth investigating. It is an Educational site with modules that well Known Oncologists have put together for the Oncology Field.
After you Signup,go to the Clinical Care Options "Oncology"
Type in Melanoma in the search field at the top of the page. You will see pages of modules to choose from.
Take your Pick!!!!
This is a great source of information.
Welcome!
Thank you for registering with the Clinical Care Options web site.
Your Current User Name is: Jimmy_b
You can change either of these by visiting http://clinicaloptions.com/ and going to "My Profile" from the web site header.
Be sure to check out:
Expert Analysis programs from major medical meetings (CME certified)
Downloadable PowerPoint Slides to use in your talks
Journal Options, a summary of clinically relevant new publications (CME certified)
We are always interested in feedback from our members.
Please email memberservices@clinicaloptions.com with any comments or questions
Jimmy B
Labels:
CTLA-4,
Dr. Flaherty,
dr. kirkwood,
interlukin-2,
Melanoma
Friday, February 6, 2009
Delayed Melanoma Response Could Impact Clinical Decisions with Anti-CTLA-4 Agents.. Melanoma..Jim Breitfller
Elsevier Global Medical News. 2008 Nov 7, JS MacNeil
STOCKHOLM (EGMN) - Clinical studies of two experimental agents - tremelimumab and ipilimumab - have shown delayed and mixed responses among patients who gained control of refractory melanomas with these therapies.
Both agents come from a new class of monoclonal antibodies that seek to promote immune response by blocking cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). In three phase II trials presented at the European Society for Medical Oncology Congress, tremelimumab and ipilimumab ultimately achieved disease control rates of 14%-29% as second-line therapies. No complete responses were reported.
Among patients classified as having progressive disease after ipilimumab treatment, however, were some people who subsequently improved. Likewise, among six patients with "mixed response" to tremelimumab were five patients who initially developed new lesions, but then had slow decreases in targeted lesions. All six were still alive at the time of the presentation.
Investigators suggested that the modified World Health Organization (WHO) criteria used to assess activity of cytotoxic agents may not capture the benefit in some patients classified with progressive disease. They cited four observed patterns of response, which were described in a poster by Dr. Kaan Harmankaya, of the department of dermatology at University of Vienna, and associates:
-Response in baseline lesions.
-Stable disease with a slow, steady decline in tumor volume.
-Response after increase in total tumor volume.
-Response in index and new lesions after the appearance of new lesions.
While delayed response is an issue for researchers, the clinical impact could be more important, according to a discussion of the tremelimumab and ipilimumab studies by Dr. Ulrich Keilholz of the Charité University in Berlin and the European Organisation for Research and Treatment of Cancer melanoma group. In comparing "classical and nonclassical efficacy assessment," he said that nonclassical assessment captures delayed efficacy, but the definitions are complicated and the issues in patient management are distinct from efficacy evaluation in studies.
"Nonclassical assessment does change the response rate, but it does not change the survival rate," Dr. Keilholz said, downplaying the importance of response measures, compared with overall survival in phase III trials.
In clinical practice, however, the physician must decide when to switch a patient from a therapy that is not working. The three trials showed that delayed efficacy does occur with these agents, but the limit appears to be week 20 after treatment, Dr. Keilholz said, adding: "I think that it is important to allow patients a certain amount of time ... but also to put a stop at a certain time point."
Tremelimumab
Dr. John M. Kirkwood, director of the Melanoma Center at the University of Pittsburgh Cancer Institute, presented the tremelimumab data from an open-label phase II trial in 251 patients (nearly all with stage IV disease), of whom 242 were evaluable. The protocol called for a 15 mg/kg dose to be delivered intravenously on the first day of up to four 12-week cycles. Sixteen (7%) patients achieved partial responses and 36 (15%) had stable disease - a clinical benefit rate of 22%.
Dr. Kirkwood said all but 1 of the partial responses lasted at least 170 days, and 11 were ongoing. Median overall survival reached 10.1 months, he said; median progression-free survival reached 2.8 months, with 15.6% of patients progression-free 6 months after treatment. Factors correlating with survival were still being analyzed. The trial was sponsored by Pfizer Inc.
Ipilimumab
The first ipilimumab trial was a multinational, open-label study of 155 patients with advanced disease that had failed previous therapies. Patients received 10 mg/kg of ipilimumab every 3 weeks for four cycles, followed by maintenance therapy at the same dose every 12 weeks from week 12 to week 60.
Dr. Vanna Chiarion Sileni of the Instituto Oncologo Veneto in Padua, Italy, reported 9 patients had partial responses and 33 had stable disease by modified WHO criteria, adding up to a disease control rate of 27% (42/155). The median duration of stable disease was 4.1 months at a median follow-up of 5.7 months, she said; 19 patients were still stable at their last assessment.
Among those classified with progressive disease were patients with the four patterns of response. Small subgroups had a "slow steady decline" in tumor volume after an initial increase in target lesions or the appearance of new lesions, she said.
In the second ipilimumab trial, Dr. Celeste Lebbé of Saint-Louis Hospital in Paris reported on a multinational dose-finding study that randomized patients with unresectable relapsed stage III or IV melanoma to 10 mg/kg, 3 mg/kg, or 0.3 mg/kg of ipilimumab given once every 3 weeks for four cycles followed by maintenance treatment once every 12 weeks.
The 10 mg/kg dose produced the best overall response rate, a composite measure of complete and partial responses, at 11%, and a disease control rate of 29% (the lowest rate, 14%, was at the lowest dose). Nearly half, 48% of 73 patients given the highest dose were alive at 1 year. Their median survival was estimated at 11 months at a median follow-up of 10.4 months.
The four patterns of response were observed in this study as well, Dr. Lebbé reported, and about 35% of patients at the highest dose had a decline in total tumor volume. Patients at this dose also had the most toxicity, she said; about a quarter had grade III adverse events, including gastrointestinal side effects in 16%. In most cases, it was manageable and reversible.
The ipilimumab studies were sponsored by Bristol-Myer Squibb and Medarex Inc, which are jointly developing the agent. Dr. Lebbé was the only investigator to disclose a conflict of interest, having served on two advisory boards for Bristol-Myer Squibb.
Dr. Kirkwood said phase III trials for both agents have been completed and are being analyzed, but applications for approval have not yet been filed. In April 2008, Pfizer halted a phase III study of tremelimumab monotherapy in advanced melanoma when interim data showed it would not be superior to standard therapy.
http://www.oncologystat.com/news-and-viewpoints/news/Delayed_Melanoma_Response_Could_Impact_Clinical_Decisions_with_Anti-CTLA-4_Agents_US.html
Delayed Melanoma Response Could Impact Clinical Decisions with Anti-CTLA-4 Agents
I believe the delayed response was what happen to me.
Jimmy B
STOCKHOLM (EGMN) - Clinical studies of two experimental agents - tremelimumab and ipilimumab - have shown delayed and mixed responses among patients who gained control of refractory melanomas with these therapies.
Both agents come from a new class of monoclonal antibodies that seek to promote immune response by blocking cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). In three phase II trials presented at the European Society for Medical Oncology Congress, tremelimumab and ipilimumab ultimately achieved disease control rates of 14%-29% as second-line therapies. No complete responses were reported.
Among patients classified as having progressive disease after ipilimumab treatment, however, were some people who subsequently improved. Likewise, among six patients with "mixed response" to tremelimumab were five patients who initially developed new lesions, but then had slow decreases in targeted lesions. All six were still alive at the time of the presentation.
Investigators suggested that the modified World Health Organization (WHO) criteria used to assess activity of cytotoxic agents may not capture the benefit in some patients classified with progressive disease. They cited four observed patterns of response, which were described in a poster by Dr. Kaan Harmankaya, of the department of dermatology at University of Vienna, and associates:
-Response in baseline lesions.
-Stable disease with a slow, steady decline in tumor volume.
-Response after increase in total tumor volume.
-Response in index and new lesions after the appearance of new lesions.
While delayed response is an issue for researchers, the clinical impact could be more important, according to a discussion of the tremelimumab and ipilimumab studies by Dr. Ulrich Keilholz of the Charité University in Berlin and the European Organisation for Research and Treatment of Cancer melanoma group. In comparing "classical and nonclassical efficacy assessment," he said that nonclassical assessment captures delayed efficacy, but the definitions are complicated and the issues in patient management are distinct from efficacy evaluation in studies.
"Nonclassical assessment does change the response rate, but it does not change the survival rate," Dr. Keilholz said, downplaying the importance of response measures, compared with overall survival in phase III trials.
In clinical practice, however, the physician must decide when to switch a patient from a therapy that is not working. The three trials showed that delayed efficacy does occur with these agents, but the limit appears to be week 20 after treatment, Dr. Keilholz said, adding: "I think that it is important to allow patients a certain amount of time ... but also to put a stop at a certain time point."
Tremelimumab
Dr. John M. Kirkwood, director of the Melanoma Center at the University of Pittsburgh Cancer Institute, presented the tremelimumab data from an open-label phase II trial in 251 patients (nearly all with stage IV disease), of whom 242 were evaluable. The protocol called for a 15 mg/kg dose to be delivered intravenously on the first day of up to four 12-week cycles. Sixteen (7%) patients achieved partial responses and 36 (15%) had stable disease - a clinical benefit rate of 22%.
Dr. Kirkwood said all but 1 of the partial responses lasted at least 170 days, and 11 were ongoing. Median overall survival reached 10.1 months, he said; median progression-free survival reached 2.8 months, with 15.6% of patients progression-free 6 months after treatment. Factors correlating with survival were still being analyzed. The trial was sponsored by Pfizer Inc.
Ipilimumab
The first ipilimumab trial was a multinational, open-label study of 155 patients with advanced disease that had failed previous therapies. Patients received 10 mg/kg of ipilimumab every 3 weeks for four cycles, followed by maintenance therapy at the same dose every 12 weeks from week 12 to week 60.
Dr. Vanna Chiarion Sileni of the Instituto Oncologo Veneto in Padua, Italy, reported 9 patients had partial responses and 33 had stable disease by modified WHO criteria, adding up to a disease control rate of 27% (42/155). The median duration of stable disease was 4.1 months at a median follow-up of 5.7 months, she said; 19 patients were still stable at their last assessment.
Among those classified with progressive disease were patients with the four patterns of response. Small subgroups had a "slow steady decline" in tumor volume after an initial increase in target lesions or the appearance of new lesions, she said.
In the second ipilimumab trial, Dr. Celeste Lebbé of Saint-Louis Hospital in Paris reported on a multinational dose-finding study that randomized patients with unresectable relapsed stage III or IV melanoma to 10 mg/kg, 3 mg/kg, or 0.3 mg/kg of ipilimumab given once every 3 weeks for four cycles followed by maintenance treatment once every 12 weeks.
The 10 mg/kg dose produced the best overall response rate, a composite measure of complete and partial responses, at 11%, and a disease control rate of 29% (the lowest rate, 14%, was at the lowest dose). Nearly half, 48% of 73 patients given the highest dose were alive at 1 year. Their median survival was estimated at 11 months at a median follow-up of 10.4 months.
The four patterns of response were observed in this study as well, Dr. Lebbé reported, and about 35% of patients at the highest dose had a decline in total tumor volume. Patients at this dose also had the most toxicity, she said; about a quarter had grade III adverse events, including gastrointestinal side effects in 16%. In most cases, it was manageable and reversible.
The ipilimumab studies were sponsored by Bristol-Myer Squibb and Medarex Inc, which are jointly developing the agent. Dr. Lebbé was the only investigator to disclose a conflict of interest, having served on two advisory boards for Bristol-Myer Squibb.
Dr. Kirkwood said phase III trials for both agents have been completed and are being analyzed, but applications for approval have not yet been filed. In April 2008, Pfizer halted a phase III study of tremelimumab monotherapy in advanced melanoma when interim data showed it would not be superior to standard therapy.
http://www.oncologystat.com/news-and-viewpoints/news/Delayed_Melanoma_Response_Could_Impact_Clinical_Decisions_with_Anti-CTLA-4_Agents_US.html
Delayed Melanoma Response Could Impact Clinical Decisions with Anti-CTLA-4 Agents
I believe the delayed response was what happen to me.
Jimmy B
Brenda Schmall Thanks for Sharing!! Melanoma Jim Breitfeller
"Please check out www.skincheck.org great information there. They will be emailing the first Melanoma Education Foundation news letters as well. Steve Fine has been doing a wonderful job with melanoma education".
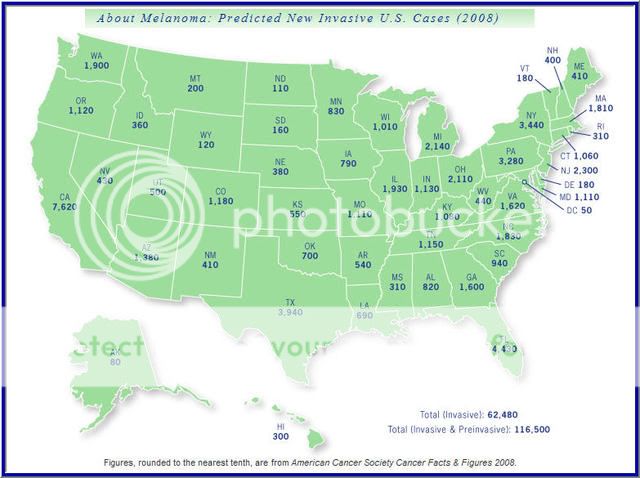
Source:Melanoma Education Foundation
http://www.skincheck.org/Page3a.htm
Please check it out.
Also, I would like to thank Brenda. Her Family was touched by Melanoma. They lost their daughter(Leanne)to the Beast at the age of 16.
Leanne web-page
Jimmy B

Source:Melanoma Education Foundation
http://www.skincheck.org/Page3a.htm
Please check it out.
Also, I would like to thank Brenda. Her Family was touched by Melanoma. They lost their daughter(Leanne)to the Beast at the age of 16.
Leanne web-page
Jimmy B
Melanoma – A Look Down The Road Ahead!! Jim Breitfeller
By Ze’ev Ronai, PhD, Burnham Institute for Medical Research, La Jolla, USA www.ronailab.net
"Malignant melanoma is one of the most highly invasive and metastatic tumors. Correspondingly, the primary clinical barriers to effective melanoma treatment are the high propensity for tumors to metastasize and the strong resistance of tumors to available treatments.
A significant advance in understanding melanoma biology was made over recent years when genetic changes along the MAPK signaling pathway (B-Raf and N-Ras mutations) were identified. Identification of these types of mutations in many melanoma tumors has significantly increased our understanding of genetic changes underlying melanoma. Yet, it is critical to note that identical genetic changes do not always underlie different melanoma subtypes. For example, mutations seen in melanomas associated with sun exposure differ from those seen in other subtypes, and some melanoma subtypes exhibit mutations in signaling pathways other than MAPK kinase. For example, c-Kit mutations were found in about 30% of mucosal, acral and other melanomas developing on chronically sun-exposed skin, conditions in which mutations in MAPK pathway components were not seen. Thus, while we have greatly advanced our understanding melanoma as a cancer marked by deregulation of well-defined signaling pathways, it is now clear that changes in multiple pathways exist among melanoma subtypes. Understanding these differences will dictate how selective therapies are developed and applied.
Adding to the complexity is the growing recognition that pathways are re-wired in melanoma. In tumors, MAPK-related pathways intersect with and influence regulatory pathways not thought to be linked in normal cells. Thus, the molecular analysis of changes in melanoma will require extensive dissection of multiple pathways influenced by deregulated genes. For example, further understanding of mechanisms underlying deregulated AKT signaling in melanoma awaits characterization.
Equally important is the growing realization that central to the etiology of melanoma is a small but potent population of melanoma initiating (stem?) cells. This exciting topic is receiving growing attention and should provide important insight into the handful of cells capable of initiating a tumor. Although currently only a few groups are dedicating their efforts to this exciting observation, an emerging theme in cancer research is that melanoma initiating cells may be as complex as melanoma tumors are.
Understanding tumor microenvironment has been and will remain critical to our understanding of melanoma progression, given that a hallmark of melanoma is its metastatic potential. The development of mouse models and better technologies to study tumor microenvironment (from 3D cultures to lymph-angiogenesis) provides tools necessary for identifying mechanisms underlying melanoma metastasis and finding selective means to halt it, as well as models to assess possible therapeutic approaches."
The rest of the article can be found at:
http://www.pigment.org/virtual.asp
Pigment Cell & Melanoma
Jimmy B
"Malignant melanoma is one of the most highly invasive and metastatic tumors. Correspondingly, the primary clinical barriers to effective melanoma treatment are the high propensity for tumors to metastasize and the strong resistance of tumors to available treatments.
A significant advance in understanding melanoma biology was made over recent years when genetic changes along the MAPK signaling pathway (B-Raf and N-Ras mutations) were identified. Identification of these types of mutations in many melanoma tumors has significantly increased our understanding of genetic changes underlying melanoma. Yet, it is critical to note that identical genetic changes do not always underlie different melanoma subtypes. For example, mutations seen in melanomas associated with sun exposure differ from those seen in other subtypes, and some melanoma subtypes exhibit mutations in signaling pathways other than MAPK kinase. For example, c-Kit mutations were found in about 30% of mucosal, acral and other melanomas developing on chronically sun-exposed skin, conditions in which mutations in MAPK pathway components were not seen. Thus, while we have greatly advanced our understanding melanoma as a cancer marked by deregulation of well-defined signaling pathways, it is now clear that changes in multiple pathways exist among melanoma subtypes. Understanding these differences will dictate how selective therapies are developed and applied.
Adding to the complexity is the growing recognition that pathways are re-wired in melanoma. In tumors, MAPK-related pathways intersect with and influence regulatory pathways not thought to be linked in normal cells. Thus, the molecular analysis of changes in melanoma will require extensive dissection of multiple pathways influenced by deregulated genes. For example, further understanding of mechanisms underlying deregulated AKT signaling in melanoma awaits characterization.
Equally important is the growing realization that central to the etiology of melanoma is a small but potent population of melanoma initiating (stem?) cells. This exciting topic is receiving growing attention and should provide important insight into the handful of cells capable of initiating a tumor. Although currently only a few groups are dedicating their efforts to this exciting observation, an emerging theme in cancer research is that melanoma initiating cells may be as complex as melanoma tumors are.
Understanding tumor microenvironment has been and will remain critical to our understanding of melanoma progression, given that a hallmark of melanoma is its metastatic potential. The development of mouse models and better technologies to study tumor microenvironment (from 3D cultures to lymph-angiogenesis) provides tools necessary for identifying mechanisms underlying melanoma metastasis and finding selective means to halt it, as well as models to assess possible therapeutic approaches."
The rest of the article can be found at:
http://www.pigment.org/virtual.asp
Pigment Cell & Melanoma
Jimmy B
Cancer Care Costs Squeeze Millions of Americans.. Melanoma..Jim Breitfeller
"Indeed, many with cancer lose their jobs. Zirkelbach cited this as a reason why individuals should consider long-term disability insurance, which would help pay bills when they are out of work because of illnesses. According to U.S. Department of Labor statistics, about 30 percent of workers in private industry -- in all, about 40 million Americans -- have employer-sponsored long-term disability insurance.
Long-term disability through private insurance can help provide some level of income for people ineligible for government programs, which require disability for a significant time. Social Security disability, which is for people who expect to be out of work temporarily but for more than a year, does not kick in until a person has been out of work because of a medical disability for at least five months. While it is rare to have delays with cancer patients, a delay in hearing time can lead to a longer waiting period for benefits. The payments, based on a complex government formula, will never be equal to job income levels.
Medicare, which is paid to people who are permanently disabled and can never return to work and covers only medical expenses, will not kick in until two years have passed since someone has started collecting Social Security disability benefits, so cancer patients who apply will not receive benefits until 29 months after their diagnosis."
Source:By JOSEPH BROWNSTEIN
ABC News Medical Unit
Feb. 5, 2009
http://abcnews.go.com/Health/OnCall/Story?id=6811555&page=1
Cancer Care Costs Squeeze Millions of Americans
Jimmy B
Long-term disability through private insurance can help provide some level of income for people ineligible for government programs, which require disability for a significant time. Social Security disability, which is for people who expect to be out of work temporarily but for more than a year, does not kick in until a person has been out of work because of a medical disability for at least five months. While it is rare to have delays with cancer patients, a delay in hearing time can lead to a longer waiting period for benefits. The payments, based on a complex government formula, will never be equal to job income levels.
Medicare, which is paid to people who are permanently disabled and can never return to work and covers only medical expenses, will not kick in until two years have passed since someone has started collecting Social Security disability benefits, so cancer patients who apply will not receive benefits until 29 months after their diagnosis."
Source:By JOSEPH BROWNSTEIN
ABC News Medical Unit
Feb. 5, 2009
http://abcnews.go.com/Health/OnCall/Story?id=6811555&page=1
Cancer Care Costs Squeeze Millions of Americans
Jimmy B
Researchers Uncover Gene for Melanoma of the Eye Jim Breitfeller
"If ever a cancer gene were discovered in the right place and at the right time, GNAQ may be it. The gene has been known for years but not linked to cancer. Now researchers say that it is mutated in patients with uveal melanoma and may be an important cause of this rare cancer, which arises from cells that give color to the eye.
The gene is in the right place because it belongs to a pathway that is activated in melanomas of the skin and has been the subject of considerable research. Drug companies are already investigating the pathway, known as the MAP kinase pathway, to develop targeted therapies for cancers other than uveal melanoma.
The time is right because of these investigations, and also because uveal melanoma may be a candidate for emerging RNA-based therapies. For example, the cancer frequently spreads through the bloodstream to the liver, and RNA delivery works particularly well in the liver.
Melanomas of the skin and the eye are biologically distinct diseases, though some of the same pathways are overactive in each. GNAQ mutations are an alternate trigger for the MAP kinase pathway, according to results published online in Nature December 10.
"Mutations in GNAQ activate a critical signaling pathway in melanoma, but it somehow enters the pathway from a different branch than melanoma of the skin," said lead investigator Dr. Boris Bastian of the University of California, San Francisco. "This may explain why the gene has not been found to be mutated in cancer until now."
GNAQ mutations were present in half of the uveal melanoma cases surveyed, and the mutations were not found in surrounding tissues.
The findings are confirmed by a follow-up study in the December Investigative Ophthalmology & Visual Science led by Dr. William Harbour of the Washington University School of Medicine.
"These authors have uncovered a mutation that certainly makes sense as a critically important mutation for uveal melanoma," said Dr. David H. Abramson, chief of ophthalmic oncology at Memorial Sloan-Kettering Cancer Center. "And what's exciting is that the discovery has the potential to go quickly from a lab experiment to a clinical treatment. Drug companies are already looking at this pathway because of other cancers."
About 1,500 cases of uveal melanoma are diagnosed each year in the United States. Although local treatment such as surgery and radiation is somewhat effective in uveal melanoma, half of the patients develop metastases to the liver even if the primary site has been effectively dealt with and controlled.
Given that some patients have metastatic disease at the time of diagnosis, what's desperately needed is a systemic therapy, said Dr. Abramson. The discovery of GNAQ represents an important step in that direction, he added.
GNAQ had been analyzed in large-scale cancer genome projects, but it went undetected as a cancer gene because the collections of melanoma samples have not been sufficiently diverse to include this rare subgroup, Dr. Bastian noted.
The project began serendipitously at a pigment cell biology meeting in 2003. Dr. Bastian heard a talk about mice developed in the laboratory of Dr. Gregory Barsh at Stanford University. The mice had a mutation that caused pigment cells to accumulate in the deep layer of the skin and resulted in bluish-black coloring on their ears and tails.
The coloring reminded Dr. Bastian of a skin condition in humans known as blue nevus, and he wondered whether the same mutations were involved. An analysis of DNA from blue nevi revealed GNAQ mutations in 80 percent of the samples. The mutation turned up only once in skin melanoma samples, however. But because a type of blue nevus is associated with a risk of uveal melanoma, the researchers looked for GNAQ mutations in that disease, and they found them.
"It's fascinating how similar the mice and the humans were in this situation," said Dr. Catherine Van Raamsdonk of the University of British Columbia, who did the analysis. "In each case, the mutations caused pigment cells to accumulate in the deep layer of the skin."
The findings raise an interesting question: Why are GNAQ mutations relatively harmless when they occur in the skin but may cause cancer in the eye? "We don't yet know why that is," said Dr. Van Raamsdonk. Benign nevi can arise within the eye, but researchers do not know whether they also carry the mutation because the nevi are not biopsied.
In recent years Dr. Bastian and others have been making the case that melanoma is actually more than one biologically distinct disease, as has been demonstrated for many cancers. The finding of GNAQ mutations in uveal melanoma and blue nevi potentially identifies them as biologically related subtypes. Mutations have also been reported in melanomas of the mucosa, palms, soles, and nail beds, suggesting that these are biologically distinct.
A critical task for the future, said Dr. Bastian, is to classify melanomas and develop therapies targeted at the genetic features underlying each form of the disease.
His laboratory is collaborating with Alnylam Pharmaceuticals to develop a therapy for silencing GNAQ in the liver. Experiments in cellas showed that introducing a small piece of genetic material (siRNA) to silence the gene caused the cancer cells to die. Alnylam has developed siRNAs for delivery to the liver in animal models, and the goal will now be to develop a panel that could selectively silence GNAQ.
Dr. Abramson praised the new studies as examples of synergy in science that does not happen often enough. "In both cases, excellent basic scientists were collaborating with excellent clinicians in work that is basic science and that could be quickly applicable to humans," he said. "That's a credit to all of them."
Source: NCI Cancer Bulletin:December 16, 2008 • Volume 5 / Number 25
Jimmy B
The gene is in the right place because it belongs to a pathway that is activated in melanomas of the skin and has been the subject of considerable research. Drug companies are already investigating the pathway, known as the MAP kinase pathway, to develop targeted therapies for cancers other than uveal melanoma.
The time is right because of these investigations, and also because uveal melanoma may be a candidate for emerging RNA-based therapies. For example, the cancer frequently spreads through the bloodstream to the liver, and RNA delivery works particularly well in the liver.
Melanomas of the skin and the eye are biologically distinct diseases, though some of the same pathways are overactive in each. GNAQ mutations are an alternate trigger for the MAP kinase pathway, according to results published online in Nature December 10.
"Mutations in GNAQ activate a critical signaling pathway in melanoma, but it somehow enters the pathway from a different branch than melanoma of the skin," said lead investigator Dr. Boris Bastian of the University of California, San Francisco. "This may explain why the gene has not been found to be mutated in cancer until now."
GNAQ mutations were present in half of the uveal melanoma cases surveyed, and the mutations were not found in surrounding tissues.
The findings are confirmed by a follow-up study in the December Investigative Ophthalmology & Visual Science led by Dr. William Harbour of the Washington University School of Medicine.
"These authors have uncovered a mutation that certainly makes sense as a critically important mutation for uveal melanoma," said Dr. David H. Abramson, chief of ophthalmic oncology at Memorial Sloan-Kettering Cancer Center. "And what's exciting is that the discovery has the potential to go quickly from a lab experiment to a clinical treatment. Drug companies are already looking at this pathway because of other cancers."
About 1,500 cases of uveal melanoma are diagnosed each year in the United States. Although local treatment such as surgery and radiation is somewhat effective in uveal melanoma, half of the patients develop metastases to the liver even if the primary site has been effectively dealt with and controlled.
Given that some patients have metastatic disease at the time of diagnosis, what's desperately needed is a systemic therapy, said Dr. Abramson. The discovery of GNAQ represents an important step in that direction, he added.
GNAQ had been analyzed in large-scale cancer genome projects, but it went undetected as a cancer gene because the collections of melanoma samples have not been sufficiently diverse to include this rare subgroup, Dr. Bastian noted.
The project began serendipitously at a pigment cell biology meeting in 2003. Dr. Bastian heard a talk about mice developed in the laboratory of Dr. Gregory Barsh at Stanford University. The mice had a mutation that caused pigment cells to accumulate in the deep layer of the skin and resulted in bluish-black coloring on their ears and tails.
The coloring reminded Dr. Bastian of a skin condition in humans known as blue nevus, and he wondered whether the same mutations were involved. An analysis of DNA from blue nevi revealed GNAQ mutations in 80 percent of the samples. The mutation turned up only once in skin melanoma samples, however. But because a type of blue nevus is associated with a risk of uveal melanoma, the researchers looked for GNAQ mutations in that disease, and they found them.
"It's fascinating how similar the mice and the humans were in this situation," said Dr. Catherine Van Raamsdonk of the University of British Columbia, who did the analysis. "In each case, the mutations caused pigment cells to accumulate in the deep layer of the skin."
The findings raise an interesting question: Why are GNAQ mutations relatively harmless when they occur in the skin but may cause cancer in the eye? "We don't yet know why that is," said Dr. Van Raamsdonk. Benign nevi can arise within the eye, but researchers do not know whether they also carry the mutation because the nevi are not biopsied.
In recent years Dr. Bastian and others have been making the case that melanoma is actually more than one biologically distinct disease, as has been demonstrated for many cancers. The finding of GNAQ mutations in uveal melanoma and blue nevi potentially identifies them as biologically related subtypes. Mutations have also been reported in melanomas of the mucosa, palms, soles, and nail beds, suggesting that these are biologically distinct.
A critical task for the future, said Dr. Bastian, is to classify melanomas and develop therapies targeted at the genetic features underlying each form of the disease.
His laboratory is collaborating with Alnylam Pharmaceuticals to develop a therapy for silencing GNAQ in the liver. Experiments in cellas showed that introducing a small piece of genetic material (siRNA) to silence the gene caused the cancer cells to die. Alnylam has developed siRNAs for delivery to the liver in animal models, and the goal will now be to develop a panel that could selectively silence GNAQ.
Dr. Abramson praised the new studies as examples of synergy in science that does not happen often enough. "In both cases, excellent basic scientists were collaborating with excellent clinicians in work that is basic science and that could be quickly applicable to humans," he said. "That's a credit to all of them."
Source: NCI Cancer Bulletin:December 16, 2008 • Volume 5 / Number 25
Jimmy B
Wednesday, February 4, 2009
Are The Europeans ahead of us in the War Against Melanoma? Jim Breitfeller
Genetics
European Organization for Research and Treatment of Cancer
EORTC Melanoma Group Genetics Committee
The mission of this sub-group is to develop genetics based translational research within the EORTC Melanoma group.
The sub-group has strong links with the melanoma genetics consortium GenoMEL @ www.genomel.org
Committee members
- Julia Newton Bishop, Chairman
- Veronique Bataille
- Brigitte Bressac
- Florence Demenais
- Giovanna Bianchi Scarra
- Dirk Schadendorf
- Johan Hansson
- Nelleke Gruis
- Susana Puig
Ongoing Studies
1. A Cohort Study of Patients with a positive sentinel node biopsy (SNB)
This study will commence recruitment in the early Autumn of 2006. Patients with a positive SNB will be offered participation in the cohort study in EORTC centres in France, Italy, the UK, Germany and Spain. The study is designed to
• Identify the histological, environmental, and genetic predictors of relapse in patients with a positive SNB so that a risk algorithm can be developed
• Increase our understanding of the biology of metastasis in melanoma patients and the genetic and environmental modifiers of that process
• Identify gene expression profiles in the primary tumour which are predictive of relapse in SNB positive patients
Participating patients will be asked to complete a questionnaire, give a blood sample from which DNA can be extracted and allow their paraffin embedded tumour tissue to be used for immunohistochemical, genetic and gene expression studies.
2. A study of inherited variation in immune response genes as modifiers of outcome in melanoma patients
Melanoma is an immunogenic tumour and it is postulated that there is inherited variation in the host’s response to the tumour, moderated by inheritance of polymorphisms in genes such as cytokine genes and the vitamin D receptor gene. Melanoma patients participating in EORTC adjuvant therapeutic trials will be asked to participate in these translational genetic studies. Consenting patients will be asked to complete a one page questionnaire based upon family history and previous medical history and to give a blood sample from which DNA can be extracted. DNA will be extracted and stored at Leiden University Medical Center, and analysed in the CR-UK Genetic Epidemiology Laboratory in Leeds and other GenoMEL laboratories. A candidate gene approach will be taken to the identification of genes hypothesized to modulate the host response to the tumour via immune processes or angiogenesis.
http://www.melanomagroup.eu/content/view/45/53/
EORTC Melanoma Group Genetics Committee
Jimmy B
European Organization for Research and Treatment of Cancer
EORTC Melanoma Group Genetics Committee
The mission of this sub-group is to develop genetics based translational research within the EORTC Melanoma group.
The sub-group has strong links with the melanoma genetics consortium GenoMEL @ www.genomel.org
Committee members
- Julia Newton Bishop, Chairman
- Veronique Bataille
- Brigitte Bressac
- Florence Demenais
- Giovanna Bianchi Scarra
- Dirk Schadendorf
- Johan Hansson
- Nelleke Gruis
- Susana Puig
Ongoing Studies
1. A Cohort Study of Patients with a positive sentinel node biopsy (SNB)
This study will commence recruitment in the early Autumn of 2006. Patients with a positive SNB will be offered participation in the cohort study in EORTC centres in France, Italy, the UK, Germany and Spain. The study is designed to
• Identify the histological, environmental, and genetic predictors of relapse in patients with a positive SNB so that a risk algorithm can be developed
• Increase our understanding of the biology of metastasis in melanoma patients and the genetic and environmental modifiers of that process
• Identify gene expression profiles in the primary tumour which are predictive of relapse in SNB positive patients
Participating patients will be asked to complete a questionnaire, give a blood sample from which DNA can be extracted and allow their paraffin embedded tumour tissue to be used for immunohistochemical, genetic and gene expression studies.
2. A study of inherited variation in immune response genes as modifiers of outcome in melanoma patients
Melanoma is an immunogenic tumour and it is postulated that there is inherited variation in the host’s response to the tumour, moderated by inheritance of polymorphisms in genes such as cytokine genes and the vitamin D receptor gene. Melanoma patients participating in EORTC adjuvant therapeutic trials will be asked to participate in these translational genetic studies. Consenting patients will be asked to complete a one page questionnaire based upon family history and previous medical history and to give a blood sample from which DNA can be extracted. DNA will be extracted and stored at Leiden University Medical Center, and analysed in the CR-UK Genetic Epidemiology Laboratory in Leeds and other GenoMEL laboratories. A candidate gene approach will be taken to the identification of genes hypothesized to modulate the host response to the tumour via immune processes or angiogenesis.
http://www.melanomagroup.eu/content/view/45/53/
EORTC Melanoma Group Genetics Committee
Jimmy B
Genomic and Molecular Profiling Predicts Response to Temozolomide in Melanoma!! Jim Breitfeller
Christina K. Augustine1,5, Jin Soo Yoo1, Anil Potti2,4, Yasunori Yoshimoto1,5, Patricia A. Zipfel1,5, Henry S. Friedman1, Joseph R. Nevins3,4, Francis Ali-Osman1 and Douglas S. Tyler1,5
Authors' Affiliations: Departments of 1 Surgery, 2 Medicine, and 3 Molecular Genetics and Microbiology, and 4 Duke Institute for Genome Sciences and Policy, Duke University Medical Center and 5 Durham VA Medical Center, Durham, North Carolina
Requests for reprints: Christina K. Augustine, Box 3118, Medical Center, Duke University Medical Center, Durham, NC 27710. Phone: 919-286-0411, ext. 5191; Fax: 919-684-6044; E-mail: Christi.augustine@duke.edu.
Purpose: Despite objective response rates of only 13%, temozolomide remains one of the most effective single chemotherapy agents against metastatic melanoma, second only to dacarbazine, the current standard of care for systemic treatment of melanoma. The goal of this study was to identify molecular and/or genetic markers that correlate with, and could be used to predict, response to temozolomide-based treatment regimens and that reflect the intrinsic properties of a patient's tumor.
Experimental Design: Using a panel of 26 human melanoma-derived cell lines, we determined in vitro temozolomide sensitivity, O6-methylguanine-DNA methyltransferase (MGMT) activity, MGMT protein expression and promoter methylation status, and mismatch repair proficiency, as well as the expression profile of 38,000 genes using an oligonucleotide-based microarray platform.
Results: The results showed a broad spectrum of temozolomide sensitivity across the panel of cell lines, with IC50 values ranging from 100 µmol/L to 1 mmol/L. There was a significant correlation between measured temozolomide sensitivity and a gene expression signature–derived prediction of temozolomide sensitivity (P < 0.005). Notably, MGMT alone showed a significant correlation with temozolomide sensitivity (MGMT activity, P < 0.0001; MGMT expression, P 0.0001). The promoter methylation status of the MGMT gene, however, was not consistent with MGMT gene expression or temozolomide sensitivity. Conclusions: These results show that melanoma resistance to temozolomide is conferred predominantly by MGMT activity and suggest that MGMT expression could potentially be a useful tool for predicting the response of melanoma patients to temozolomide therapy. Source: Clinical Cancer Research 15, 502-510, January 15, 2009. doi: 10.1158/1078-0432.CCR-08-1916 http://clincancerres.aacrjournals.org/cgi/content/short/15/2/502
Authors' Affiliations: Departments of 1 Surgery, 2 Medicine, and 3 Molecular Genetics and Microbiology, and 4 Duke Institute for Genome Sciences and Policy, Duke University Medical Center and 5 Durham VA Medical Center, Durham, North Carolina
Requests for reprints: Christina K. Augustine, Box 3118, Medical Center, Duke University Medical Center, Durham, NC 27710. Phone: 919-286-0411, ext. 5191; Fax: 919-684-6044; E-mail: Christi.augustine@duke.edu.
Purpose: Despite objective response rates of only 13%, temozolomide remains one of the most effective single chemotherapy agents against metastatic melanoma, second only to dacarbazine, the current standard of care for systemic treatment of melanoma. The goal of this study was to identify molecular and/or genetic markers that correlate with, and could be used to predict, response to temozolomide-based treatment regimens and that reflect the intrinsic properties of a patient's tumor.
Experimental Design: Using a panel of 26 human melanoma-derived cell lines, we determined in vitro temozolomide sensitivity, O6-methylguanine-DNA methyltransferase (MGMT) activity, MGMT protein expression and promoter methylation status, and mismatch repair proficiency, as well as the expression profile of 38,000 genes using an oligonucleotide-based microarray platform.
Results: The results showed a broad spectrum of temozolomide sensitivity across the panel of cell lines, with IC50 values ranging from 100 µmol/L to 1 mmol/L. There was a significant correlation between measured temozolomide sensitivity and a gene expression signature–derived prediction of temozolomide sensitivity (P < 0.005). Notably, MGMT alone showed a significant correlation with temozolomide sensitivity (MGMT activity, P < 0.0001; MGMT expression, P 0.0001). The promoter methylation status of the MGMT gene, however, was not consistent with MGMT gene expression or temozolomide sensitivity. Conclusions: These results show that melanoma resistance to temozolomide is conferred predominantly by MGMT activity and suggest that MGMT expression could potentially be a useful tool for predicting the response of melanoma patients to temozolomide therapy. Source: Clinical Cancer Research 15, 502-510, January 15, 2009. doi: 10.1158/1078-0432.CCR-08-1916 http://clincancerres.aacrjournals.org/cgi/content/short/15/2/502
'Targeted Chemo' Safely Shrinks Liver Tumors ..Melanoma..Jim Breitfeller
"Linda Campbell had already beaten ocular melanoma, a rare form of skin cancer, or so she thought. Then during the summer, doctors discovered the tumors had spread to her liver.
Using new technology, doctors thread a tube up to the liver and inject the high doses of the chemotherapy drug directly to the cancer site for thirty minutes.
(ABCNews Photo Illustration)"There were so many they couldn't count them," said Campbell.
Campbell, who's 59 years old and lives with her husband outside of Lexington, N.C., learned that when cancer spreads to the liver, it becomes much more deadly.
It does not matter whether cancer starts in the breast or colon, lung or skin, the real damage is caused when it metastasizes to the liver. Every year an estimated 200,000 Americans receive the grim diagnosis and few patients survive more than a year.
"There was a lot of crying. ... And we weren't sure what to do," Campbell told ABC News..
Doctors suggested Campbell enroll in a clinical trial testing a new, radically different type of chemotherapy. This experimental technique targets only the cancer site, allowing doctors to flood the liver with 10 times the dose of a standard chemotherapy drug."
Source: ABC News
I believe a story will be on tonight with Charles Gibson on ABC
Cutting Edge Therapy
Jimmy B
Using new technology, doctors thread a tube up to the liver and inject the high doses of the chemotherapy drug directly to the cancer site for thirty minutes.
(ABCNews Photo Illustration)"There were so many they couldn't count them," said Campbell.
Campbell, who's 59 years old and lives with her husband outside of Lexington, N.C., learned that when cancer spreads to the liver, it becomes much more deadly.
It does not matter whether cancer starts in the breast or colon, lung or skin, the real damage is caused when it metastasizes to the liver. Every year an estimated 200,000 Americans receive the grim diagnosis and few patients survive more than a year.
"There was a lot of crying. ... And we weren't sure what to do," Campbell told ABC News..
Doctors suggested Campbell enroll in a clinical trial testing a new, radically different type of chemotherapy. This experimental technique targets only the cancer site, allowing doctors to flood the liver with 10 times the dose of a standard chemotherapy drug."
Source: ABC News
I believe a story will be on tonight with Charles Gibson on ABC
Cutting Edge Therapy
Jimmy B
Labels:
Delcath,
liver Tumor,
Melanoma,
Ocular Melanoma,
Video-link
Targeted Therapy ...Ocular/unveal Melanoma ..Jim Breitfeller
As I continue my series on Targeted Therapy, I was contacted and was asked to put the word out for Ocular Melanoma Patients. Well I did not know very much about this cancer. So, I decided to research it before I said anything.
About 2000 people get diagnosed with Ocular Melanoma a year. Ocular melanoma is a lethal and very rare disease. Many people die from it, especially when it spreads to the liver, a common complication. There are other types of eye cancers, but melanoma is the most common.
Ocular melanoma, a rare cancer, is a disease in which cancer (malignant) cells are found in the part of the eye called the uvea. The uvea contains cells called melanocytes. When these cells become cancerous, the cancer is called a melanoma. The uvea includes the iris (the colored part of the eye), the ciliary body (a muscle in the eye), and the choroid (a layer of tissue in the back of the eye). The iris opens and closes to change the amount of light entering the eye. The ciliary body changes the shape of the lens inside the eye so it can focus. The choroid layer is next to the retina, the part of the eye that makes a picture. If there is melanoma that starts in the iris, it may look like a dark spot on the iris. If melanoma is in the ciliary body or the choroid, a person may have blurry vision or may have no symptoms, and the cancer may grow before it is noticed. Intraocular melanoma is usually found during a routine eye examination, when a doctor looks inside the eye with special lights and instruments.1
The chance of recovery (prognosis) depends on the size and cell type of the cancer, where the cancer is in the eye, and whether the cancer has spread.
What are the Symptoms?
“Ocular melanoma may not cause any early symptoms. It is sometimes found during a routine eye exam when the doctor dilates the pupil and looks into the eye. The following symptoms may be caused by intraocular melanoma or by other conditions. A doctor should be consulted if any of these problems occur:”2
• A dark spot on the iris.
• Blurred vision.
• A change in the shape of the pupil.
• A change in vision.
If it is caught early, it is curable. Left untreated this melanoma usually metastasizes to the liver and onto other sites. It is very difficult to treat once it has metastasize. Uveal melanoma can be effectively treated, but once it metastasizes to
the liver, survival can be less than 6 months.
What are the Known Treatments?
A good web site to go for information from Patients like us is:
http://www.lafn.org/~bc534/OcularMelMets.htm
Uveal Melanoma Metastatic to the Liver
There are at least five different treatments for Ocular (uveal) melanoma patients who have liver metastases.
Immunoembolization
“Immunoembolization is a variation of chemoembolization, which involves infusing a
cytotoxic agent into a tumor after mechanically or surgically disrupting the tumor's
blood supply. The advantage of immunoembolization is that it
could attract and stimulate antigen-presenting cells in liver tumors and improve the
uptake of tumor antigens released from necrotic tumor cells, as well as potentially
facilitate a systemic immune response against tumor cells and thereby suppress the
growth of extrahepatic metastases.”3
Stereotactic Body Radiosurgery
“Stereotactic body radiosurgery(SBR) is a novel treatment that can be employed in the treatment of metastatic melanoma. Stereotactic body radiosurgery is a treatment that uses multiple high powered beams aimed very precisely at tumors in the body. The Elekta frame immobilizes the patient and decreases the movement of the liver and lung tumors due to breathing. The treatment spares normal tissue because of its extreme precision. The treatment is completely non invasive and is given in 1-3 outpatient treatments. Patients can continue there normal daily activites as there are often minimal side effects. Metastic melanoma, melanoma that has spread to other organs, is a difficult situation because there is not effective drug therapy. Occasionally, if possible, the tumors can be surgically removed. However, surgery is usually not possible and can be quite morbid with extensive recovery times. Much as surgery, Stereotactic body radiosurgery can eradicate treated tumors in the lung and liver 90 percent of the time. Unlike surgery, there is very little risk and minimal side effects. Patients are able to continue their daily activities without difficulty. The high tumor eradication rate is a result of its high dose per treatment.
Patient with recurrent metastatic melanoma or diagnosed initially with metastatic melanoma are potential candidates. Patients need to have relatively few liver or lung metastasis and have good funtional status. Some doctors have arbitrarily set the limit to 5 tumors.”4
Radiofrequency Ablation
“Radiofrequency ablation (RFA) is an exciting technique used to treat malignant liver tumors. An electrical current is transmitted through a small needle placed directly into a liver tumor, to destroy cancer cells with heat. An array of hook electrodes are deployed from the tip of the needle after it's placed in the tumor. The hook electrodes look like the ribs of an umbrella, with a diameter of 3.5 centimeters (slightly greater than an inch) when fully deployed (figure 1). An ultrasound probe is used to guide placement of the needle, and after the needle is in the correct area, the hook electrodes are deployed into the liver tumor. The treatment is started by applying electrical current from a small, briefcase-sized device called a radiofrequency current generator. The amount of power supplied by the generator can be controlled precisely, and the generator is also used to monitor the treatment until complete heat-induced destruction of the tumor being treated occur.”
“The key in each of these patients is that the liver was the only cancer site. We do not perform RFA in patients with cancer that has spread to other areas or organs, because treating only the liver will not improve these patients’ chances for survival. We also have learned that it is difficult, and probably not helpful, to treat patients with more than eight tumors, or if more than half of their liver is replaced by tumors. Lastly, we find that the RFA equipment currently available does not reliably destroy all the tumor if it is larger than six centimeters in diameter. These larger tumors should be removed when possible. Thus, we do not recommend RFA for these larger liver tumors, but we, and others, are working to develop RFA systems that will effectively treat larger tumors. 5”
Chemoembolization.
A procedure in which the blood supply to the tumor is blocked surgically or mechanically and anticancer drugs are administered directly into the tumor. This permits a higher concentration of drug to be in contact with the tumor for a longer period of time. Unlike conventional systemic chemotherapy, the drugs injected through the hepatic artery are confined to the liver. Side effects of chemotherapy are minimized as a result.
Isolated Liver Perfusion
This treatment for liver tumors was first described over thirty years ago but has had limited clinical use until now. The basis of this treatment is to expose the liver to high doses of chemotherapy in order to achieve maximal tumor shrinkage. The blood supply to the liver is completely isolated from the systemic circulation so that the body is not exposed to the high dose of drugs. It requires a lengthy operation to completely mobilize the liver and to insert catheters into the hepatic artery, portal vein and hepatic veins.
Recently, initial results using different drugs have been encouraging and work continues with this modality. This is experimental therapy and should only be used in a clinical trial.
Patients with isolated hepatic metastasis from Ocular Melanoma are indeed rare. In the largest prospective data base on melanoma in the world (the combined experience of The John Wayne Cancer Institute and Sydney Melanoma Unit) only 24 of 1750 patients (1.3%) with hepatic metastasis were found to have disease that was resectable6
Since the most of the patients would have non-resectable with hepatic metastasis, I have been contacted by Delcath Systems that is the sponsor of the trial.
“I oversee Delcath Systems' National Cancer Institute-led Phase III clinical trial for metastatic melanoma to the liver. As you know, there are few effective treatment options for patients once melanoma has metastasized to the liver, however, we are highly encouraged by our current clinical trial, which showed tremendous response rates in its corresponding Phase I study. We are treating patients at a number of cancer centers throughout the country using a minimally invasive procedure that targets the liver for high dose drug treatment. In addition to overseeing the operations of this trial, I am also trying to increase the awareness of its availability to patients, medical oncologists, surgical oncologists, dermatologists and other physicians.”
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
NEW YORK, November 18, 2008 – Delcath Systems, Inc. (Nasdaq: DCTH), a medical technology company testing its proprietary liver cancer treatment for melanoma metastatic to the liver, announced today that the United States Food and Drug Administration (“FDA”) granted to Delcath two orphan-drug applications for the drug melphalan. Delcath was granted designations of the drug melphalan for the treatment of patients with cutaneous as well as ocular melanoma. Approximately 65,000 cases of these melanomas are diagnosed annually in the United States.
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver. This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Source: Delcath Systems Inc.
So, I went out and tried to contact the Oncologists that are participating in the trial.
http://www.delcath.com/news/2008/treatmentcenters.html
Current Trial Centers
Oncologist’s Comments:
“We are participating in the percutaneous hepatic perfusion with melphalan trial. This treatment is for patients with melanoma metastatic to the liver (either from the eye or skin), with little or no melanoma elsewhere. The response rates so far are high (the majority of patients respond in the liver) and patients have tolerated the perfusion well. The main risks have to do with the thinning of the blood that occurs during the perfusion and the possibility of the chemotherapy escaping the isolated area and affecting other parts of the body, but problems have been rare. The main disadvantage is that the rest of the body is not treated, only the liver. This is fine if the areas of melanoma are restricted to the liver exclusively, or almost exclusively. Obviously there is a lot more to it, but I’d be happy to give more information if anyone had specific questions.”
“One good thing about the perfusion trial is that, though it is randomized, there is the potential for “crossover”. That is, patients are assigned to get the perfusion or get the best alternative care (almost any alternative is allowed), and if their liver disease grows on the other therapy, they can switch over to the perfusion. If it were me, I would do the perfusion trial if I had unresectable, liver only metastases.
The leakage is rare and has not happened at all to my knowledge since some protocol changes were implemented.
Every patient is different and I would encourage everyone to explore options and go to a melanoma center where the most options can be considered.”
“Because this is an active ongoing Phase III clinical trial there is no preliminary data to give. What I can tell you is that the technique and the phase I data was published in a the Journal of Clinical Oncology in May 2005. Because we did have some great responses in melanoma patients, we are testing this technique against the best available therapy. This technique is best used for patients with disease confined to the liver but there are exceptions to this.”
As usual, I was in contact with Dr. James Pingpank, author and principle investigator of:
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancie.
He was encouraged with the results so far.
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
My Take on the subject as I see it:
This very hard disease to treat.
This Therapy is not a cure all, but it seems to extend the survival for most patients while maintaining a good quality of life base on the disease.
As one Oncologist said “Every patient is different and I would encourage everyone to explore options and go to a melanoma center where the most options can be considered.”
References
1. http://www.kellogg.umich.edu/patientcare/conditions/intraocular.melanoma.html
2. http://www.cancer.gov/cancertopics/pdq/treatment/intraocularmelanoma/Patient#Keypoint3
3. Immunoembolization safe, promising for liver metastases from uveal melanoma October 24, 2008 American Society of Clinical Oncology
4. http://www.cancertreatmentgroup.com/metastatic_melanoma.shtml
5. http://www.mdanderson.org/departments/ltsg/display.cfm?id=6193B8E8-BC8D-11D4-80FB00508B603A14&pn=9DF6DAEE-7AAF-430F-8ADC4CAF62712136&method=displayfull
6. Rose DM, Essner R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg 2001; 136: 950–5.
Take care
Jimmy B
Melanoma_Missionary
About 2000 people get diagnosed with Ocular Melanoma a year. Ocular melanoma is a lethal and very rare disease. Many people die from it, especially when it spreads to the liver, a common complication. There are other types of eye cancers, but melanoma is the most common.
Ocular melanoma, a rare cancer, is a disease in which cancer (malignant) cells are found in the part of the eye called the uvea. The uvea contains cells called melanocytes. When these cells become cancerous, the cancer is called a melanoma. The uvea includes the iris (the colored part of the eye), the ciliary body (a muscle in the eye), and the choroid (a layer of tissue in the back of the eye). The iris opens and closes to change the amount of light entering the eye. The ciliary body changes the shape of the lens inside the eye so it can focus. The choroid layer is next to the retina, the part of the eye that makes a picture. If there is melanoma that starts in the iris, it may look like a dark spot on the iris. If melanoma is in the ciliary body or the choroid, a person may have blurry vision or may have no symptoms, and the cancer may grow before it is noticed. Intraocular melanoma is usually found during a routine eye examination, when a doctor looks inside the eye with special lights and instruments.1
The chance of recovery (prognosis) depends on the size and cell type of the cancer, where the cancer is in the eye, and whether the cancer has spread.
What are the Symptoms?
“Ocular melanoma may not cause any early symptoms. It is sometimes found during a routine eye exam when the doctor dilates the pupil and looks into the eye. The following symptoms may be caused by intraocular melanoma or by other conditions. A doctor should be consulted if any of these problems occur:”2
• A dark spot on the iris.
• Blurred vision.
• A change in the shape of the pupil.
• A change in vision.
If it is caught early, it is curable. Left untreated this melanoma usually metastasizes to the liver and onto other sites. It is very difficult to treat once it has metastasize. Uveal melanoma can be effectively treated, but once it metastasizes to
the liver, survival can be less than 6 months.
What are the Known Treatments?
A good web site to go for information from Patients like us is:
http://www.lafn.org/~bc534/OcularMelMets.htm
Uveal Melanoma Metastatic to the Liver
There are at least five different treatments for Ocular (uveal) melanoma patients who have liver metastases.
Immunoembolization
“Immunoembolization is a variation of chemoembolization, which involves infusing a
cytotoxic agent into a tumor after mechanically or surgically disrupting the tumor's
blood supply. The advantage of immunoembolization is that it
could attract and stimulate antigen-presenting cells in liver tumors and improve the
uptake of tumor antigens released from necrotic tumor cells, as well as potentially
facilitate a systemic immune response against tumor cells and thereby suppress the
growth of extrahepatic metastases.”3
Stereotactic Body Radiosurgery
“Stereotactic body radiosurgery(SBR) is a novel treatment that can be employed in the treatment of metastatic melanoma. Stereotactic body radiosurgery is a treatment that uses multiple high powered beams aimed very precisely at tumors in the body. The Elekta frame immobilizes the patient and decreases the movement of the liver and lung tumors due to breathing. The treatment spares normal tissue because of its extreme precision. The treatment is completely non invasive and is given in 1-3 outpatient treatments. Patients can continue there normal daily activites as there are often minimal side effects. Metastic melanoma, melanoma that has spread to other organs, is a difficult situation because there is not effective drug therapy. Occasionally, if possible, the tumors can be surgically removed. However, surgery is usually not possible and can be quite morbid with extensive recovery times. Much as surgery, Stereotactic body radiosurgery can eradicate treated tumors in the lung and liver 90 percent of the time. Unlike surgery, there is very little risk and minimal side effects. Patients are able to continue their daily activities without difficulty. The high tumor eradication rate is a result of its high dose per treatment.
Patient with recurrent metastatic melanoma or diagnosed initially with metastatic melanoma are potential candidates. Patients need to have relatively few liver or lung metastasis and have good funtional status. Some doctors have arbitrarily set the limit to 5 tumors.”4
Radiofrequency Ablation
“Radiofrequency ablation (RFA) is an exciting technique used to treat malignant liver tumors. An electrical current is transmitted through a small needle placed directly into a liver tumor, to destroy cancer cells with heat. An array of hook electrodes are deployed from the tip of the needle after it's placed in the tumor. The hook electrodes look like the ribs of an umbrella, with a diameter of 3.5 centimeters (slightly greater than an inch) when fully deployed (figure 1). An ultrasound probe is used to guide placement of the needle, and after the needle is in the correct area, the hook electrodes are deployed into the liver tumor. The treatment is started by applying electrical current from a small, briefcase-sized device called a radiofrequency current generator. The amount of power supplied by the generator can be controlled precisely, and the generator is also used to monitor the treatment until complete heat-induced destruction of the tumor being treated occur.”
“The key in each of these patients is that the liver was the only cancer site. We do not perform RFA in patients with cancer that has spread to other areas or organs, because treating only the liver will not improve these patients’ chances for survival. We also have learned that it is difficult, and probably not helpful, to treat patients with more than eight tumors, or if more than half of their liver is replaced by tumors. Lastly, we find that the RFA equipment currently available does not reliably destroy all the tumor if it is larger than six centimeters in diameter. These larger tumors should be removed when possible. Thus, we do not recommend RFA for these larger liver tumors, but we, and others, are working to develop RFA systems that will effectively treat larger tumors. 5”
Chemoembolization.
A procedure in which the blood supply to the tumor is blocked surgically or mechanically and anticancer drugs are administered directly into the tumor. This permits a higher concentration of drug to be in contact with the tumor for a longer period of time. Unlike conventional systemic chemotherapy, the drugs injected through the hepatic artery are confined to the liver. Side effects of chemotherapy are minimized as a result.
Isolated Liver Perfusion
This treatment for liver tumors was first described over thirty years ago but has had limited clinical use until now. The basis of this treatment is to expose the liver to high doses of chemotherapy in order to achieve maximal tumor shrinkage. The blood supply to the liver is completely isolated from the systemic circulation so that the body is not exposed to the high dose of drugs. It requires a lengthy operation to completely mobilize the liver and to insert catheters into the hepatic artery, portal vein and hepatic veins.
Recently, initial results using different drugs have been encouraging and work continues with this modality. This is experimental therapy and should only be used in a clinical trial.
Patients with isolated hepatic metastasis from Ocular Melanoma are indeed rare. In the largest prospective data base on melanoma in the world (the combined experience of The John Wayne Cancer Institute and Sydney Melanoma Unit) only 24 of 1750 patients (1.3%) with hepatic metastasis were found to have disease that was resectable6
Since the most of the patients would have non-resectable with hepatic metastasis, I have been contacted by Delcath Systems that is the sponsor of the trial.
“I oversee Delcath Systems' National Cancer Institute-led Phase III clinical trial for metastatic melanoma to the liver. As you know, there are few effective treatment options for patients once melanoma has metastasized to the liver, however, we are highly encouraged by our current clinical trial, which showed tremendous response rates in its corresponding Phase I study. We are treating patients at a number of cancer centers throughout the country using a minimally invasive procedure that targets the liver for high dose drug treatment. In addition to overseeing the operations of this trial, I am also trying to increase the awareness of its availability to patients, medical oncologists, surgical oncologists, dermatologists and other physicians.”
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
NEW YORK, November 18, 2008 – Delcath Systems, Inc. (Nasdaq: DCTH), a medical technology company testing its proprietary liver cancer treatment for melanoma metastatic to the liver, announced today that the United States Food and Drug Administration (“FDA”) granted to Delcath two orphan-drug applications for the drug melphalan. Delcath was granted designations of the drug melphalan for the treatment of patients with cutaneous as well as ocular melanoma. Approximately 65,000 cases of these melanomas are diagnosed annually in the United States.
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver. This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Source: Delcath Systems Inc.
So, I went out and tried to contact the Oncologists that are participating in the trial.
http://www.delcath.com/news/2008/treatmentcenters.html
Current Trial Centers
Oncologist’s Comments:
“We are participating in the percutaneous hepatic perfusion with melphalan trial. This treatment is for patients with melanoma metastatic to the liver (either from the eye or skin), with little or no melanoma elsewhere. The response rates so far are high (the majority of patients respond in the liver) and patients have tolerated the perfusion well. The main risks have to do with the thinning of the blood that occurs during the perfusion and the possibility of the chemotherapy escaping the isolated area and affecting other parts of the body, but problems have been rare. The main disadvantage is that the rest of the body is not treated, only the liver. This is fine if the areas of melanoma are restricted to the liver exclusively, or almost exclusively. Obviously there is a lot more to it, but I’d be happy to give more information if anyone had specific questions.”
“One good thing about the perfusion trial is that, though it is randomized, there is the potential for “crossover”. That is, patients are assigned to get the perfusion or get the best alternative care (almost any alternative is allowed), and if their liver disease grows on the other therapy, they can switch over to the perfusion. If it were me, I would do the perfusion trial if I had unresectable, liver only metastases.
The leakage is rare and has not happened at all to my knowledge since some protocol changes were implemented.
Every patient is different and I would encourage everyone to explore options and go to a melanoma center where the most options can be considered.”
“Because this is an active ongoing Phase III clinical trial there is no preliminary data to give. What I can tell you is that the technique and the phase I data was published in a the Journal of Clinical Oncology in May 2005. Because we did have some great responses in melanoma patients, we are testing this technique against the best available therapy. This technique is best used for patients with disease confined to the liver but there are exceptions to this.”
As usual, I was in contact with Dr. James Pingpank, author and principle investigator of:
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancie.
He was encouraged with the results so far.
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
My Take on the subject as I see it:
This very hard disease to treat.
This Therapy is not a cure all, but it seems to extend the survival for most patients while maintaining a good quality of life base on the disease.
As one Oncologist said “Every patient is different and I would encourage everyone to explore options and go to a melanoma center where the most options can be considered.”
References
1. http://www.kellogg.umich.edu/patientcare/conditions/intraocular.melanoma.html
2. http://www.cancer.gov/cancertopics/pdq/treatment/intraocularmelanoma/Patient#Keypoint3
3. Immunoembolization safe, promising for liver metastases from uveal melanoma October 24, 2008 American Society of Clinical Oncology
4. http://www.cancertreatmentgroup.com/metastatic_melanoma.shtml
5. http://www.mdanderson.org/departments/ltsg/display.cfm?id=6193B8E8-BC8D-11D4-80FB00508B603A14&pn=9DF6DAEE-7AAF-430F-8ADC4CAF62712136&method=displayfull
6. Rose DM, Essner R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg 2001; 136: 950–5.
Take care
Jimmy B
Melanoma_Missionary
Subscribe to:
Comments (Atom)
Greetings to One and All
This Blog is dedicated My Brother Kenny B. who passed away in the late 1970's with Cancer before the Internet.
It was he, who showed me How to live and give back. He was wise beyond his years.

Jimmy and Dee
Carepage: Jimmybreitfeller
Jimmy Breitfeller
It was he, who showed me How to live and give back. He was wise beyond his years.

Jimmy and Dee
Carepage: Jimmybreitfeller
Jimmy Breitfeller
My Profile as of 2009

- jimmy_B
- Last July (2005)I was riding my bicycle to work at the Eastman Kodak Research Labs about 3 miles from home. I was wearing a knapsack to carry my things to and from the labs. I started noticing an ache on my back. So I decide to go to the dermatologist. To make the long story short, it was cancer. I knew from my research that I would be needing adjuvant therapy. So I started communicating with Sloan Kettering, University of Pittsburgh Cancer Center, and a couple of others including the Wilmot Cancer Center at Strong. I realized that by telling my story, I might help someone else out there in a similar situation. So to all who are linked by diagnosis or by relation to someone with melanoma, I wish you well. Stay positive, read as much as you can (information helps to eliminate the fear associated with the unknown), and live for today, as no one can predict what tomorrow may bring. Jimmy B. posted 12/15/08
Disclaimer
The information contained within this Blog is not meant to replace the examination or advice of your Oncologist or Medical Team. The educational material that is covered here or Linked to, does not cover every detail of each disorder discussed.
Only your physician/Oncologist can make medical decisions and treatment plans that are appropriate for you. But, An Educated Consumer is a Smart consumer.
As Dr. Casey Culberson Said:
"The BEST melanoma patient is an ACTIVE PARTICIPANT in his or her treatment
(not a PASSIVE RECIPIENT)"
Only your physician/Oncologist can make medical decisions and treatment plans that are appropriate for you. But, An Educated Consumer is a Smart consumer.
As Dr. Casey Culberson Said:
"The BEST melanoma patient is an ACTIVE PARTICIPANT in his or her treatment
(not a PASSIVE RECIPIENT)"
Melanoma and the “Magic Bullet” (Monoclonal Antibodies)
Just to let you know I posted the first draft of the Melanoma and the “Magic Bullet” (Monoclonal Antibodies). on Melanoma Missionary In the Shared File Section. you can download it for 19.95 (Only kidding) it is Free for the taking.
It is 33 pages long and may help you in your quest for the Yellow Brick Broad. Just to let you know it is only the first draft. Revisions are sure to come. I wanted to get it to the people that need it the most, the Melanoma Patients.
Preview:
So, where does Interluekin-2 (IL-2) come into play? According to Byung-Scok et al and recent reports, IL-2 is not needed for developmental CD4+ CD25+ Treg cells in the thymus but does play an important role in the maintenance and function in the peripheral.18 Peripheral is defines as secondary system outside the bone marrow and thymus. It entails the site of antigen, immune system interaction. IL-2 is required for the peripheral generation of Tregs based Abbas’s and colleagues research.19
IL-2 prevents the spontaneous apoptosis of the CD4+ CD25+ Treg cells. It has been reported that patients with multiple advance-stage tumors have elevated levels of Tregs within the tumor microenviroment.20 Interluekin-2 is the survival factor for CD4+ CD25+ Treg cells.21 If the addition of IL-2 is on or before the maximum propagation of the CD4+ T cells, the Tregs population can increase 5-fold in a 96 hour period based on certain growth mediums.
By controlling the addition of the endogenous IL-2, one has a knob to turn and can lead to the control of the expansion of the Tregs. When you combined this control with the anti-CTLA-4 blockage, you can shift the balance of the immune response.
Now here is the catch. The maintenance and function of the CD8+ T-cells require CD4+ cells which secrete IL-2. So we don’t want to deplete the CD4+ cells, we want to control the expansion of the Tregs which are a subset of the CD4+ cells. It has been postulated by some researchers that the Anti-CTLA-4 blockage also suppresses the Treg function in a different mechanism. By using IL-2 as the rate limiting factor, we can suppress the CD4+ CD25+ Treg cell expansion by controlling the concentration and timing of the Inerluekin-2 at the tumor microenvironment.
The Interluekin-2 plays another role in this Melanoma Maze. In a study by Janas et al, Il-2 increases the expressions of the perforin and granzyme A, B and C genes in the CD8+ T-cells. This increase expression causes the CD8+ T-cells to mature into Cytoxic T Lymphocytes (CTLs). The exogenous IL-2 is required for the granzyme proteins. As stated previously, CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Once in the tumor cell, these enzymes are able to breakup (lyse) the cell and destroy it. This is the beginning of the end for the cancer cells. The tumors begin to shrink and the rest is history,
“
On the other hand, prolong therapy with Il-2 can result in causing apoptotic death of the tumor- specific CD8+ T-cells.23
Clearly in a clinical setting, timing, dose, and exposure to these drugs play a major roll in the immunotherapy, and can have dramatic effects on the outcome.
All it takes is that one magic bullet to start the immune reaction..
https://app.box.com/shared/kjgr6dkztj
Melanoma And The Magic Bullet (Monoclonal Antibodies)
It is 33 pages long and may help you in your quest for the Yellow Brick Broad. Just to let you know it is only the first draft. Revisions are sure to come. I wanted to get it to the people that need it the most, the Melanoma Patients.
Preview:
So, where does Interluekin-2 (IL-2) come into play? According to Byung-Scok et al and recent reports, IL-2 is not needed for developmental CD4+ CD25+ Treg cells in the thymus but does play an important role in the maintenance and function in the peripheral.18 Peripheral is defines as secondary system outside the bone marrow and thymus. It entails the site of antigen, immune system interaction. IL-2 is required for the peripheral generation of Tregs based Abbas’s and colleagues research.19
IL-2 prevents the spontaneous apoptosis of the CD4+ CD25+ Treg cells. It has been reported that patients with multiple advance-stage tumors have elevated levels of Tregs within the tumor microenviroment.20 Interluekin-2 is the survival factor for CD4+ CD25+ Treg cells.21 If the addition of IL-2 is on or before the maximum propagation of the CD4+ T cells, the Tregs population can increase 5-fold in a 96 hour period based on certain growth mediums.
By controlling the addition of the endogenous IL-2, one has a knob to turn and can lead to the control of the expansion of the Tregs. When you combined this control with the anti-CTLA-4 blockage, you can shift the balance of the immune response.
Now here is the catch. The maintenance and function of the CD8+ T-cells require CD4+ cells which secrete IL-2. So we don’t want to deplete the CD4+ cells, we want to control the expansion of the Tregs which are a subset of the CD4+ cells. It has been postulated by some researchers that the Anti-CTLA-4 blockage also suppresses the Treg function in a different mechanism. By using IL-2 as the rate limiting factor, we can suppress the CD4+ CD25+ Treg cell expansion by controlling the concentration and timing of the Inerluekin-2 at the tumor microenvironment.
The Interluekin-2 plays another role in this Melanoma Maze. In a study by Janas et al, Il-2 increases the expressions of the perforin and granzyme A, B and C genes in the CD8+ T-cells. This increase expression causes the CD8+ T-cells to mature into Cytoxic T Lymphocytes (CTLs). The exogenous IL-2 is required for the granzyme proteins. As stated previously, CTLs have cytoplasmic granules that contain the proteins perforin and granzymes. A dozen or more perforin molecules insert themselves into the plasma membrane of target cells forming a pore that enables granzymes to enter the cell. Once in the tumor cell, these enzymes are able to breakup (lyse) the cell and destroy it. This is the beginning of the end for the cancer cells. The tumors begin to shrink and the rest is history,
“
On the other hand, prolong therapy with Il-2 can result in causing apoptotic death of the tumor- specific CD8+ T-cells.23
Clearly in a clinical setting, timing, dose, and exposure to these drugs play a major roll in the immunotherapy, and can have dramatic effects on the outcome.
All it takes is that one magic bullet to start the immune reaction..
https://app.box.com/shared/kjgr6dkztj
Melanoma And The Magic Bullet (Monoclonal Antibodies)
Public Service Announcement
A call for Melanoma Patients by Dr. Steven A Rosenberg
"We continue to see a high rate of clinical responses in our cell transfer immunotherapy treatments for patients with metastatic melanoma", Dr. Rosenberg said.
"We are actively seeking patients for these trials and any note of that on a patient-directed web site would be appreciated."
If you would like to apply for his trials, here is the website and information.
Dr. Rosenberg's information
Dr. Rosenberg's Clinical Trials

The Melanoma Research Alliance has partnered with Bruce Springsteen, the E Street Band, and the Federici family to alleviate suffering and death from melanoma. Please view Bruce Springsteen’s public service announcement inspired by Danny Federici. Danny was the E Street Band’s organist and keyboard player. He died on April 17, 2008 at Memorial Sloan-Kettering Cancer Center in New York City after a three year battle with melanoma.
http://www.melanomaresearchalliance.org/news/PSA/
Source Fastcures blog
"We continue to see a high rate of clinical responses in our cell transfer immunotherapy treatments for patients with metastatic melanoma", Dr. Rosenberg said.
"We are actively seeking patients for these trials and any note of that on a patient-directed web site would be appreciated."
If you would like to apply for his trials, here is the website and information.
Dr. Rosenberg's information
Dr. Rosenberg's Clinical Trials

The Melanoma Research Alliance has partnered with Bruce Springsteen, the E Street Band, and the Federici family to alleviate suffering and death from melanoma. Please view Bruce Springsteen’s public service announcement inspired by Danny Federici. Danny was the E Street Band’s organist and keyboard player. He died on April 17, 2008 at Memorial Sloan-Kettering Cancer Center in New York City after a three year battle with melanoma.
http://www.melanomaresearchalliance.org/news/PSA/
Source Fastcures blog
Join the Relay for Life!!!

Dear Family and Friends,
I’ve decided to take a stand and fight back against cancer by participating in the American Cancer Society Relay For Life® event right here in my community! Please support me in this important cause by making a secure, tax-deductible donation online using the link below.
To donate on line now, click here to visit my personal page.
Jimmy B AKA Melanoma_Missionary
Relay For Life® is a life-changing event that brings together more than 3.5 million people worldwide to:
CELEBRATE the lives of those who have battled cancer. The strength of survivors inspires others to continue to fight.
REMEMBER loved ones lost to the disease. At Relay, people who have walked alongside people battling cancer can grieve and find healing.
FIGHT BACK. We Relay because we have been touched by cancer and desperately want to put an end to the disease.
Whatever you can give will help - it all adds up! I greatly appreciate your support and will keep you posted on my progress.
Keep the Fire Burning!!!

Sincerely,
Jimmy Breitfeller
Turn off Music before you "Click to Play"
Signs of Melanoma Carcinoma Skin Cancer
Signs of Melanoma Carcinoma Skin Cancer
How Skin Cancer Develops by "About.com : Dermatology"
Call for Patients with Unresectable Liver Metastases Due to Melanoma
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver.
This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Current Trial Centers
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
Delcath Systems Granted Orphan-Drug Designations for Cutaneous and Ocular Melanoma
Delcath is actively enrolling patients in a Phase III clinical trial testing its proprietary drug delivery system, known as Percutaneous Hepatic Perfusion (“PHP”), with melphalan for the treatment of ocular and cutaneous melanoma metastatic to the liver.
This NCI-led trial is enrolling patients at leading cancer centers throughout the United States. Commenting on these orphan-drug designations, Richard L. Taney, President and CEO of Delcath, stated, “These favorable designations are important steps in our efforts to secure Delcath’s commercial position upon conclusion of our pivotal Phase III trial for metastatic melanoma. We remain steadfast in our commitment to become the leader in the regional treatment of liver cancers and we continue to enroll patients in this study, and advance our technology and the promise that it offers to patients with these deadly forms of melanoma and other cancers of the liver, all with limited treatment options.”
Orphan drug designation, when granted by the FDA’s Office of Orphan Products Development, allows for up to seven years of market exclusivity upon FDA approval, as well as clinical study incentives, study design assistance, waivers of certain FDA user fees, and potential tax credits.
Current Trial Centers
Phase I Study of Hepatic Arterial Melphalan Infusion and Hepatic Venous Hemofiltration Using
Percutaneously Placed Catheters in Patients With Unresectable Hepatic Malignancies
James F. Pingpank, Jr., MD, FACS
Associate Professor of Surgery
Division of Surgical Oncology
Suite 406, UPMC Cancer Pavillion
5150 Centre Avenue
Pittsburgh, PA 15232
412-692-2852 (Office)
412-692-2520 (Fax)
PingpankJF@UPMC.edu
Blog Archive
-
▼
2009
(332)
-
▼
February
(45)
- Did you have your Blueberries Today??Melanoma ..Ji...
- Cancer Miracles !!!!!!! Melanoma Jim Breitfeller
- Hand and Hand Tumor Rejection ..Melanoma ..Jim Bre...
- Jay Tenenbaum Urges Collaboration To Treat the Lon...
- Fewer, Smaller Skin Cancer Tumors After Blocking P...
- In Loving Memory ..Melanoma.. Jim Breitfeller
- IL-2 Regulates Perforin and Granzyme Gene Expressi...
- I AM STUCK AT BASE CAMP 2!!! Melanoma Jim Breitfeller
- Are You Going to Finish Strong? Melanoma Jim Breit...
- Patient knowledge of health information influences...
- Failure at the effector phase: immune barriers at ...
- Here Goes!!! I am going to Spill the Beans!!!!!! M...
- Next Slide Please!!!!!!! Melanoma Jim Breitfeller
- Natural Killer T-cells!!!!! Melanoma Jim Breitfeller
- We are all in it together!!!!!! And the Beat goes...
- Patty Luker, This for you!!! Melanoma..Jim Breitfe...
- I believe I am Half way There!!!!!!!! Melanoma Jim...
- CTLA-4 Therapy--Permission to used Diagrams..Melan...
- Helpful hint of the day!!!!!!!! Melanoma.. Jim Bre...
- Dr. Polly Matzinger Danger!! Danger!! Melanoma..Ji...
- Genomics: The convoluted promise of our generation...
- Figure 1. T-cell Mobilized!!! Melanoma..Jim Breitf...
- If you are wondering where I am!!!!! Melanoma .. J...
- A Conversation with Dr. Natalie Ahn ..Melanoma..Ji...
- A Stage IV Malignant Melanoma Drug That Increases ...
- Micro RNA Plays A Key Role In Melanoma Metastasis....
- Did the clinical trials on CTLA-4 Therapy Sell us ...
- Remember I said HOMEWORK..HOMEWORK..HOMEWORK!!!!! ...
- Clinical Care Options!!! Melanoma Jim Breitfeller
- Delayed Melanoma Response Could Impact Clinical De...
- Brenda Schmall Thanks for Sharing!! Melanoma Jim B...
- Melanoma – A Look Down The Road Ahead!! Jim Breitf...
- Cancer Care Costs Squeeze Millions of Americans.. ...
- Researchers Uncover Gene for Melanoma of the Eye J...
- Are The Europeans ahead of us in the War Against M...
- Genomic and Molecular Profiling Predicts Response ...
- 'Targeted Chemo' Safely Shrinks Liver Tumors ..Mel...
- Targeted Therapy ...Ocular/unveal Melanoma ..Jim ...
- I am back from my Punch Biopsy !!!! Melanoma .. Ji...
- Cambridge University Research Group Employs Fluidi...
- Stress May Hasten Growth Of Melanoma Tumors!! Jim ...
- You heard it here first..“Personalized medicine is...
- Subject: BRAF V600E mutational analysis 7...
- Oncogene Inhibits Tumor Suppressor To Promote Canc...
- Trends in Melanoma -----Sanjiv. S. Aqarwala, MD Ja...
-
▼
February
(45)
Call For Melanoma Patients!!!!
Call For Melanoma Patients!!!!
Dr. Rosenberg Has a New Clinical Trial.
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Dr. Rosenberg's Clinical Trials
(For a copy of the research paper.. see My Shared files)
Dr. Rosenberg Has a New Clinical Trial.
Our latest treatment has a 72% objective response rate with 36% complete responses.
We are currently recruiting patients for our latest trial.
Is there some way to post this “Call for Patients” on the web site?
Steve Rosenberg
Dr. Rosenberg's Clinical Trials
(For a copy of the research paper.. see My Shared files)
The news headlines shown above for Melanoma / Skin Cancer are provided courtesy of Medical News Today.


